Genetic Ablation of Extra Domain A of Fibronectin in Hypercholesterolemic Mice Improves Stroke Outcome by Reducing Thrombo-Inflammation
- PMID: 26508731
- PMCID: PMC4674336
- DOI: 10.1161/CIRCULATIONAHA.115.016540
Genetic Ablation of Extra Domain A of Fibronectin in Hypercholesterolemic Mice Improves Stroke Outcome by Reducing Thrombo-Inflammation
Abstract
Background: The fibronectin-splicing variant containing extra domain A (Fn-EDA) is present in negligible amounts in the plasma of healthy humans but markedly elevated in patients with comorbid conditions, including diabetes mellitus and hypercholesterolemia, which are risk factors for stroke. It remains unknown, however, whether Fn-EDA worsens stroke outcomes in such conditions. We determined the role of Fn-EDA in stroke outcome in a model of hypercholesterolemia, the apolipoprotein E-deficient (Apoe(-/-)) mouse.
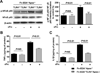
Methods and results: In a transient cerebral ischemia/reperfusion injury model, Apoe(-/-) mice expressing fibronectin deficient in EDA (Fn-EDA(-/-)Apoe(-/-) mice) exhibited smaller infarcts and improved neurological outcomes at days 1 and 8 (P<0.05 versus Apoe(-/-) mice). Concomitantly, intracerebral thrombosis [assessed by fibrin(ogen) deposition] and postischemic inflammation (phospho-nuclear factor-κB p65, phospho-IκB kinase α/β, interleukin 1β, and tumor necrosis factor-α) within lesions of Fn-EDA(-/-)Apoe(-/-) mice were markedly decreased (P<0.05 versus Apoe(-/-) mice). In an FeCl3 injury-induced carotid artery thrombosis model, thrombus growth rate and the time to occlusion were prolonged in Fn-EDA(-/-)Apoe(-/-) mice (P<0.05 versus Apoe(-/-) mice). Genetic ablation of TLR4 improved stroke outcome in Apoe(-/-) mice (P<0.05) but had no effect on stroke outcome in Fn-EDA(-/-)Apoe(-/-) mice. Bone marrow transplantation experiments revealed that nonhematopoietic cell-derived Fn-EDA exacerbates stroke through Toll-like receptor-4 expressed on hematopoietic cells. Infusion of a specific inhibitor of Fn-EDA into Apoe(-/-) mouse 15 minutes after reperfusion significantly improved stroke outcome.
Conclusions: Hypercholesterolemic mice deficient in Fn-EDA exhibit reduced cerebral thrombosis and less inflammatory response after ischemia/reperfusion injury. These findings suggest that targeting Fn-EDA could be an effective therapeutic strategy in stroke associated with hypercholesterolemia.
Keywords: fibronectins; hypercholesterolemia; inflammation; stroke; thrombosis.
© 2015 American Heart Association, Inc.
Figures


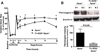







Comment in
-
Toward a Biological Therapy to Improve Stroke Outcomes After Thrombolytic Therapy.Circulation. 2015 Dec 8;132(23):2201-2. doi: 10.1161/CIRCULATIONAHA.115.019446. Epub 2015 Oct 27. Circulation. 2015. PMID: 26508732 No abstract available.
References
-
- Warach S, Latour LL. Evidence of reperfusion injury, exacerbated by thrombolytic therapy, in human focal brain ischemia using a novel imaging marker of early blood-brain barrier disruption. Stroke. 2004;35:2659–2661. - PubMed
-
- Aronowski J, Strong R, Grotta JC. Reperfusion injury: Demonstration of brain damage produced by reperfusion after transient focal ischemia in rats. J Cereb Blood Flow Metab. 1997;17:1048–1056. - PubMed
-
- Tan MH, Sun Z, Opitz SL, Schmidt TE, Peters JH, George EL. Deletion of the alternatively spliced fibronectin eiiia domain in mice reduces atherosclerosis. Blood. 2004;104:11–18. - PubMed
-
- Lefebvre JS, Levesque T, Picard S, Pare G, Gravel A, Flamand L, Borgeat P. Extra domain a of fibronectin primes leukotriene biosynthesis and stimulates neutrophil migration through activation of toll-like receptor 4. Arthritis Rheum. 2011;63:1527–1533. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

