A human leucyl-tRNA synthetase as an anticancer target
- PMID: 26508878
- PMCID: PMC4610879
- DOI: 10.2147/OTT.S88873
A human leucyl-tRNA synthetase as an anticancer target
Abstract
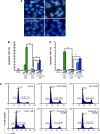
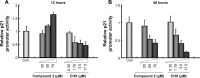
Several aminoacyl-tRNA synthetases have been reported to be overexpressed for charging essential aminoacyl-tRNAs in many cancer types. In this study, we aimed to explore the potential role of leucyl-tRNA synthetase (LARS) as an anticancer target. MTT assay was performed to screen inhibitors to human LARS (hsLARS) from compounds AN2690 and its derivatives, compounds 1-6, in U2OS and SKOV3 cells. The compound with the strongest inhibitory ability was further investigated for its inhibitory effect in cancer cell lines and in an animal tumor model. Additionally, a LARS-rescue experiment was performed to explore the potential target in U2OS using Western blot and flow cytometry. Luciferase reporter assay was designed to analyze the effect of of hsLARS inhibitor on p21 activation. We identified an hsLARS inhibitor (compound 2) that suppressed the proliferation of U2OS and SKOV3 cells in vitro. A LARS-rescue experiment demonstrated that the proliferation inhibition was induced by targeting intracellular LARS. In addition, the hsLARS inhibition was shown to activate the p21 early transcription and promote cell apoptosis, as well as reduce implanted EMT6 tumor progression in mice. Our results suggest that LARS might serve as a potential anticancer target through the p21 signaling pathway and that the nutritional signaling pathway may provide a valuable anticancer strategy for further investigation.
Keywords: anticancer target; apoptosis; leucyl-tRNA synthetase; mouse model.
Figures



References
-
- Medina MA, Marquez J, de Castro IN. Interchange of amino acids between tumor and host. Biochem Med Metab Biol. 1992;48(1):1–7. - PubMed
-
- Giese C, Lepthien S, Metzner L, Brandsch M, Budisa N, Lilie H. Intracellular uptake and inhibitory activity of aromatic fluorinated amino acids in human breast cancer cells. Chem Med Chem. 2008;3(9):1449–1456. - PubMed
-
- Shikano N, Ogura M, Okudaira H, et al. Uptake of 3-[125I]iodo-alpha-methyl-L-tyrosine into colon cancer DLD-1 cells: characterization and inhibitory effect of natural amino acids and amino acid-like drugs. Nucl Med Biol. 2010;37(2):197–204. - PubMed
-
- Cascino A, Muscaritoli M, Cangiano C, et al. Plasma amino acid imbalance in patients with lung and breast cancer. Anticancer Res. 1995;15(2):507–510. - PubMed
-
- Nishihira T, Takagi T, Kawarabayashi Y, et al. Anti-cancer therapy with valine-depleted amino acid imbalance solution. Tohoku J Exp Med. 1988;156(3):259–270. - PubMed
LinkOut - more resources
Full Text Sources

