Age, Tumor Characteristics, and Treatment Regimen as Event Predictors in Ewing: A Children's Oncology Group Report
- PMID: 26508901
- PMCID: PMC4609872
- DOI: 10.1155/2015/927123
Age, Tumor Characteristics, and Treatment Regimen as Event Predictors in Ewing: A Children's Oncology Group Report
Abstract
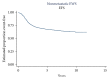
Purpose. To associate baseline patient characteristics and relapse across consecutive COG studies. Methods. We analyzed risk factors for LESFT patients in three randomized COG trials. We evaluated age at enrollment, primary site, gender, tumor size, and treatment (as randomized). We estimated event-free survival (EFS, Kaplan-Meier) and compared risk across groups (log-rank test). Characteristics were assessed by proportional hazards regression with the characteristic of interest as the only component. Confidence intervals (CI) for RR were derived. Factors related to outcome at level 0.05 were included in a multivariate regression model. Results. Between 12/1988 and 8/2005, 1444 patients were enrolled and data current to 2001, 2004, or 2008 were used. Patients were with a median age of 12 years (0-45), 55% male and 88% Caucasian. The 5-year EFS was 68.3% ± 1.3%. In univariate analysis age, treatment, and tumor location were identified for inclusion in the multivariate model, and all remained significant (p < 0.01). Since tumor size was not collected in the last study, the other two were reanalyzed. This model identified age, treatment, tumor location, and tumor size as significant predictors. Conclusion. Age > 18 years, pelvic tumor, size > 8 cms, and chemotherapy without ifosfamide/etoposide significantly predict worse outcome. AEWS0031 is NCT00006734, INT0091 and INT0054 designed before 1993 (unregistered).
Figures
References
-
- Craft A., Cotterill S., Malcolm A., et al. Ifosfamide-containing chemotherapy in Ewing's sarcoma: the second United Kingdom Children's Cancer Study Group and the Medical Research Council Ewing's Tumor Study. Journal of Clinical Oncology. 1998;16(11):3628–3633. - PubMed
-
- Womer R. B., West D. C., Krailo M. D., et al. Randomized controlled trial of interval-compressed chemotherapy for the treatment of localized ewing sarcoma: a report from the children's oncology group. Journal of Clinical Oncology. 2012;30(33):4148–4154. doi: 10.1200/jco.2011.41.5703. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

