Recurrent Thrombotic Events after Discontinuation of Vitamin K Antagonist Treatment for Splanchnic Vein Thrombosis: A Multicenter Retrospective Cohort Study
- PMID: 26508913
- PMCID: PMC4609867
- DOI: 10.1155/2015/620217
Recurrent Thrombotic Events after Discontinuation of Vitamin K Antagonist Treatment for Splanchnic Vein Thrombosis: A Multicenter Retrospective Cohort Study
Abstract
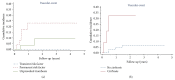
It is generally recommended that patients with splanchnic vein thrombosis (SVT) should receive a minimum of 3 months of anticoagulant treatment. However, little information is available on the long-term risk of recurrent thrombotic events. The aim of this study was to evaluate the risk of venous and arterial thrombosis after discontinuation of vitamin K antagonist (VKA) in SVT patients. Retrospective information from a cohort of SVT patients treated with VKA and followed by 37 Italian Anticoagulation Clinics, up to June 2013, was collected. Only patients who discontinued VKA and did not receive any other anticoagulant drug were enrolled in this study. Thrombotic events during follow-up were centrally adjudicated. Ninety patients were included: 33 unprovoked SVT, 27 SVT secondary to transient risk factors, and 30 with permanent risk factors. During a median follow-up of 1.6 years, 6 venous and 1 arterial thrombosis were documented, for an incidence of 3.3/100 patient-years (pt-y). The recurrence rate was highest in the first year after VKA discontinuation (8.2/100'pt-y) and in patients with permanent risk factors (10.2/100'pt-y). Liver cirrhosis significantly increased the risk of recurrence. In conclusion, the rate of recurrent vascular complications after SVT is not negligible, at least in some patient subgroups.
Figures

References
LinkOut - more resources
Full Text Sources
Other Literature Sources

