Patient-reported outcomes as end points in clinical trials in rheumatoid arthritis
- PMID: 26509052
- PMCID: PMC4613162
- DOI: 10.1136/rmdopen-2014-000019
Patient-reported outcomes as end points in clinical trials in rheumatoid arthritis
Abstract
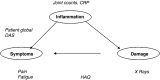
There is a growing interest in patient-reported outcomes (PROs) in rheumatology, which goes with a global trend for more 'patient-centred care'. This review considers the use of PROs in trials, including their strengths and limitations. In rheumatoid arthritis (RA) trials, the most frequently used PROs to assess treatments include pain, patient global assessment, assessment of functional status, but also health-related quality of life and less commonly fatigue. Other aspects of importance for patients, such as sleep, psychological well-being or ability to cope, are rarely assessed. PROs as outcome measures in RA trials have strengths as well as limitations. PROs have face validity, they are reproducible and sensitive to change and they bring additional information beyond joint counts or acute phase reactants. However, their predictive validity for later outcomes has been little explored, some PROs show redundancy (they bring similar information) and, due to the apparently moderate link between some PROs such as fatigue and the disease process, the use of some PROs to inform treatment choices has been questioned. We suggest the choice of PROs for trials depends on the study objective and on the viewpoint of the stakeholder. There needs to be agreed prioritisation across all stakeholders about what is most important to collect in a trial, which is why a prioritisation and selection process is necessary. Trials in RA will continue to include PROs and their interpretation will become easier as our knowledge progresses.
Keywords: Outcomes research; Patient perspective; Rheumatoid Arthritis.
Figures
References
-
- http://handbook.cochrane.org/chapter_17/17_patient_reported_outcomes.htm (accessed 16 Feb 2015).
-
- Kirwan JR, Heiberg T, Hewlett SA. Outcomes from the Patient Perspective Workshop at OMERACT 6. J Rheumatol 2003;30:868–72. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous