Biological treatment in systemic juvenile idiopathic arthritis: achievement of inactive disease or clinical remission on a first, second or third biological agent
- PMID: 26509061
- PMCID: PMC4613174
- DOI: 10.1136/rmdopen-2014-000036
Biological treatment in systemic juvenile idiopathic arthritis: achievement of inactive disease or clinical remission on a first, second or third biological agent
Abstract
Objectives: To analyse the effect of biological agents (BAs) in terms of achieving inactive disease (ID) or clinical remission (CR) in patients with systemic juvenile idiopathic arthritis (SJIA), to describe effects of switching or discontinuing a BA and to assess the proportion of patients able to maintain ID or CR off steroids and after withdrawing BA therapy.
Methods: Retrospective study in a French paediatric rheumatology reference centre using the CEMARA (CEntre des MAladies RAres) register.
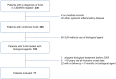
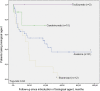
Results: Seventy-seven patients were included with a cumulative follow-up of 245.5 patient-years (median 1.1, range 0.5-8.0). On a first BA, ID was achieved in 37 patients, including 1 patient out of 12 patients on etanercept, 26 patients out of 51 on anakinra and 7 out of 10 on canakinumab. One patient on abatacept and two patients on tocilizumab also achieved ID. Switching of BA was common. The switch to a second (n=34), third (n=18) or fourth (n=4) BA resulted in ID in a further 13 patients, either on canakinumab (n=6) or tocilizumab (n=7). At last follow-up, 40 patients were in CR (27 patients off steroids, 5 patients having never received steroid treatment), either on (n=29) or off (n=11) BA.
Conclusions: In this series of patients with SJIA, interleukin-1 inhibitors were associated with a higher proportion of ID than tumour necrosis factor inhibitors when used as first BA. Switching allowed some patients to achieve ID when treated with canakinumab or tocilizumab. CR was eventually achieved in more than half of the patients.
Keywords: DMARDs (biologic); Juvenile Idiopathic Arthritis; Outcomes research; TNF-alpha.
Figures

References
-
- Petty RE, Southwood TR, Manners P et al. . International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol 2004;31:390–2. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
