Delineating the Tes Interaction Site in Zyxin and Studying Cellular Effects of Its Disruption
- PMID: 26509500
- PMCID: PMC4624954
- DOI: 10.1371/journal.pone.0140511
Delineating the Tes Interaction Site in Zyxin and Studying Cellular Effects of Its Disruption
Abstract
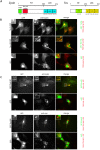
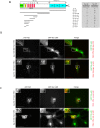
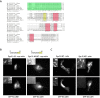
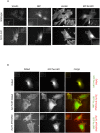
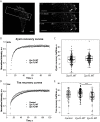
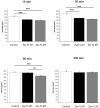
Focal adhesions are integrin-based structures that link the actin cytoskeleton and the extracellular matrix. They play an important role in various cellular functions such as cell signaling, cell motility and cell shape. To ensure and fine tune these different cellular functions, adhesions are regulated by a large number of proteins. The LIM domain protein zyxin localizes to focal adhesions where it participates in the regulation of the actin cytoskeleton. Because of its interactions with a variety of binding partners, zyxin has been proposed to act as a molecular scaffold. Here, we studied the interaction of zyxin with such a partner: Tes. Similar to zyxin, Tes harbors three highly conserved LIM domains of which the LIM1 domain directly interacts with zyxin. Using different zyxin variants in pull-down assays and ectopic recruitment experiments, we identified the Tes binding site in zyxin and showed that four highly conserved amino acids are crucial for its interaction with Tes. Based upon these findings, we used a zyxin mutant defective in Tes-binding to assess the functional consequences of abrogating the zyxin-Tes interaction in focal adhesions. Performing fluorescence recovery after photobleaching, we showed that zyxin recruits Tes to focal adhesions and modulates its turnover in these structures. However, we also provide evidence for zyxin-independent localization of Tes to focal adhesions. Zyxin increases focal adhesion numbers and reduces focal adhesion lifetimes, but does so independent of Tes. Quantitative analysis showed that the loss of interaction between zyxin and Tes affects the process of cell spreading. We conclude that zyxin influences focal adhesion dynamics, that it recruits Tes and that this interaction is functional in regulating cell spreading.
Conflict of interest statement
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

