Bloch-Siegert B1+-mapping for human cardiac (31) P-MRS at 7 Tesla
- PMID: 26509652
- PMCID: PMC5076794
- DOI: 10.1002/mrm.26005
Bloch-Siegert B1+-mapping for human cardiac (31) P-MRS at 7 Tesla
Abstract
Purpose: Phosphorus MR spectroscopy ((31) P-MRS) is a powerful tool for investigating tissue energetics in vivo. Cardiac (31) P-MRS is typically performed using surface coils that create an inhomogeneous excitation field across the myocardium. Accurate measurements of B1+ (and hence flip angle) are necessary for quantitative analysis of (31) P-MR spectra. We demonstrate a Bloch-Siegert B1+-mapping method for this purpose.
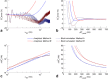
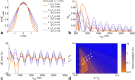
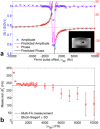
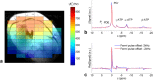
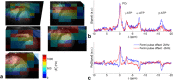
Theory and methods: We compare acquisition strategies for Bloch-Siegert B1+-mapping when there are several spectral peaks. We optimize a Bloch-Siegert sensitizing (Fermi) pulse for cardiac (31) P-MRS at 7 Tesla (T) and apply it in a three-dimensional (3D) chemical shift imaging sequence. We validate this in phantoms and skeletal muscle (against a dual-TR method) and present the first cardiac (31) P B1+-maps at 7T.
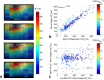
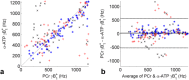
Results: The Bloch-Siegert method correlates strongly (Pearson's r = 0.90 and 0.84) and has bias <25 Hz compared with a multi-TR method in phantoms and dual-TR method in muscle. Cardiac 3D B1+-maps were measured in five normal volunteers. B1+ maps based on phosphocreatine and alpha-adenosine-triphosphate correlated strongly (r = 0.62), confirming that the method is T1 insensitive.
Conclusion: The 3D (31) P Bloch-Siegert B1+-mapping is consistent with reference methods in phantoms and skeletal muscle. It is the first method appropriate for (31) P B1+-mapping in the human heart at 7T. Magn Reson Med 76:1047-1058, 2016. © 2015 The Authors. Magnetic Resonance in Medicine published by Wiley Periodicals, Inc. on behalf of International Society for Magnetic Resonance in Medicine. This is an open access article under the terms of the Creative Commons Attribution License, which permits use, distribution and reproduction in any medium, provided the original work is properly cited.
Keywords: 31P-MRS; 7T; B1+-mapping; Bloch-Siegert; cardiac; heart; phosphorus; spectroscopy.
© 2015 The Authors. Magnetic Resonance in Medicine published by Wiley Periodicals, Inc. on behalf of International Society for Magnetic Resonance in Medicine.
Figures


References
-
- Bottomley PA. NMR spectroscopy of the human heart In: Harris RK, Wasylishen RE, editors. Encylcopedia of magnetic resonance. Chichester: John Wiley; 2009.
-
- Neubauer S. Mechanisms of disease ‐ the failing heart ‐ an engine out of fuel. N Engl J Med 2007;356:1140–1151. - PubMed
-
- Hudsmith LE, Neubauer S. Detection of myocardial disorders by magnetic resonance spectroscopy. Nat Clin Pract Card 2008;5:S49–S56. - PubMed
-
- Neubauer S, Horn M, Cramer M, et al. Myocardial phosphocreatine‐to‐ATP ratio is a predictor of mortality in patients with dilated cardiomyopathy. Circulation 1997;96:2190–2196. - PubMed
-
- Stoll V, Clarke WT, Levelt E, Myerson SG, Robson MD, Neubauer S, Rodgers CT. 7T versus 3T phosphorus magnetic resonance spectroscopy in patients with dilated cardiomyopathy. J Cardiovasc Magn Reson 2015;17(Suppl. 1):249.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

