Lithium for schizophrenia
- PMID: 26509923
- PMCID: PMC6984626
- DOI: 10.1002/14651858.CD003834.pub3
Lithium for schizophrenia
Abstract
Background: Many people with schizophrenia do not achieve a satisfactory treatment response with ordinary anti-psychotic drug treatment. In these cases, various add-on medications are used, among them lithium.
Objectives: To assess whether:1. Lithium alone is an effective treatment for schizophrenia, schizophrenia-like psychoses and schizoaffective psychoses; and2. Lithium augmentation of antipsychotic medication is an effective treatment for the same illnesses.
Search methods: In July 2012, we searched the Cochrane Schizophrenia Group's Study-Based Register of Trials which is based on regular searches of CINAHL, BIOSIS, AMED, EMBASE, PubMed, MEDLINE, PsycINFO, and registries of clinical trials. This search was updated on January 20, 2015. For the first version of the review, we also contacted pharmaceutical companies and authors of relevant studies to identify further trials and obtain original participant data.
Selection criteria: Randomised controlled trials (RCTs) of lithium compared with antipsychotics or placebo (or no intervention), whether as sole treatment or as an adjunct to antipsychotic medication, in the treatment of schizophrenia or schizophrenia-like psychoses or both.
Data collection and analysis: We extracted data independently. For dichotomous data, we calculated random-effects meta-analyses, risk ratios (RRs), and 95% confidence intervals (CI) on an intention-to-treat basis. For continuous data, we calculated mean differences (MD) and 95% confidence intervals. We used Grading of Recommendations Assessment, Development and Evaluation (GRADE) to create 'Summary of findings' tables and assessed risk of bias for included studies.
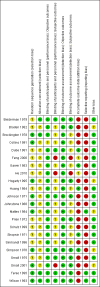
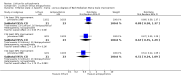
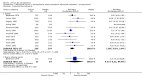
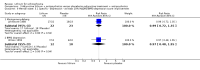
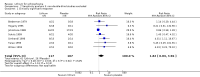
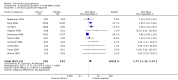
Main results: The update search in 2012 detected two further studies that met our inclusion criteria. We did not find any further studies that met our inclusion criteria in the 2015 search. This review now includes 22 studies, with a total of 763 participants (median mean age: 35 years, range: 26 to 72 years). Most studies were small, of short duration, and incompletely reported. As we detected a high risk of bias in many studies, the overall methodological quality of the included sample was rather low.Three small studies comparing lithium with placebo as the sole treatment showed no difference in any of the outcomes we analysed.In eight studies comparing lithium with antipsychotic drugs as the sole treatment, more participants in the lithium group left the studies early (eight RCTs; n = 270, RR 1.77, 95% CI 1.01 to 3.11, low quality evidence).Thirteen studies examined whether the augmentation of antipsychotic drugs with lithium salts is more effective than antipsychotic drugs alone. More participants who received lithium augmentation had a clinically significant response (10 RCTs; n = 396, RR 1.81, 95% CI 1.10 to 2.97, low quality evidence). However, this effect became non-significant when we excluded participants with schizoaffective disorders in a sensitivity analysis (seven RCTs; n = 272, RR 1.64, 95% CI 0.95 to 2.81), when we excluded non-double-blind studies (seven RCTs; n = 224, RR 1.82, 95% CI 0.84 to 3.96), or when we excluded studies with high attrition (nine RCTs; n = 355, RR 1.67, CI 0.93 to 3.00). The overall acceptability of treatment (measured by the number of participants leaving the studies early) was not significantly different between groups (11 RCTs; n = 320, RR 1.89, CI 0.93 to 3.84, very low quality evidence). Few studies reported on side effects. There were no significant differences, but the database is too limited to make any judgement in this regard. For example, there were no data on thyroid dysfunction and kidney problems - two major and well-known side effects of lithium.
Authors' conclusions: The evidence base for the use of lithium in schizophrenia is limited to 22 studies of overall low methodological quality. There is no randomised trial-based evidence that lithium on its own is an effective treatment for people with schizophrenia. There is some GRADE low quality evidence that augmentation of antipsychotics with lithium is effective, but the effects are not significant when more prone-to-bias open RCTs are excluded. Nevertheless, further large and well-designed trials are justified. These should concentrate on two target groups: (1) people with no affective symptoms, so that trialists can determine whether lithium has an effect on the core symptoms of schizophrenia, and (2) people with schizoaffective disorders for whom lithium is widely used in clinical practice, although there is no evidence to support this use.
Conflict of interest statement
Stefan Leucht: in the last three years Stefan Leucht has received honoraria for lectures from EliLilly, Lundbeck (Institute), Pfizer, Janssen, BMS, Johnson and Johnson, Otsuka, Roche, SanofiAventis, ICON, Abbvie, AOP Orphan, Servier; for consulting/advisory boards from Roche, Janssen, Lundbeck, EliLilly, Otsuka, TEVA; for the preparation of educational material and publications from Lundbeck Institute and Roche. EliLilly has provided medication for a clinical trial led by SL as principal investigator. John McGrath: John McGrath is a member of the following advisory boards: Eli Lilly Australia, Lundbeck Australia, and Pfizer Australia. In addition, JM has been a co‐investigator on studies of neuroleptic medications produced by the following companies: Astra, Janssen‐Cilag, Eli Lilly, Zeneca (ICI), Sandoz, and Pfizer. The same companies have provided travel and accommodation expenses for John McGrath to attend relevant investigator meetings and scientific symposia. No funds have been paid directly to Dr McGrath. Payments related to participation in drug trials and board attendances have been paid to a government‐audited trust account to support schizophrenia research. Werner Kissling: Werner Kissling has received honoraria or research support or both from Janssen‐Cilag, Sanofi‐Aventis, Johnson & Johnson, Pfizer, Bristol‐Myers Squibb, AstraZeneca, Lundbeck, Novartis, and Eli Lilly. Markus Dold: nothing to declare. Bartosz Helfer: nothing to declare.
Figures





























































Update of
-
Lithium for schizophrenia.Cochrane Database Syst Rev. 2007 Jul 18;(3):CD003834. doi: 10.1002/14651858.CD003834.pub2. Cochrane Database Syst Rev. 2007. Update in: Cochrane Database Syst Rev. 2015 Oct 28;(10):CD003834. doi: 10.1002/14651858.CD003834.pub3. PMID: 17636738 Updated.
References
References to studies included in this review
Biederman 1979 {published data only}
-
- Biederman J, Lerner Y, Belmaker RH. Combination of lithium carbonate and haloperidol in schizo‐affective patients. Archives of General Psychiatry 1979 Mar;36:327‐33. - PubMed
Braden 1982 {published data only}
-
- Braden W, Fink EB, Qualls CB, Ho CK, Samuels WO. Lithium and chlorpromazine in psychotic inpatients. Psychiatry Research 1982;7:69‐81. - PubMed
Brockington 1978 {published data only}
-
- Brockington I, Kendell R, Kellett J, Curry S, Wainwright S. Trials of lithium, chlorpromazine and amitriptyline in schizoaffective patients. British Journal of Psychiatry 1978 Aug;133:162‐8. - PubMed
Collins 1991 {published data only}
-
- Collins EJ, Larkins EP, Shubsacs AP. Lithium carbonate in chronic schizophrenia ‐ a brief trial of lithium carbonated added to neuroleptics for treatment resistant schizophrenic patients. Acta Psychiatrica Scandinavica 1991;84:150‐4. - PubMed
Dube 1981 {published data only}
Feng 2006 {published data only}
-
- Feng Z, Yang K‐J, Gao H, Wu D‐H, Wu H‐A. The effect of lithium carbonate combining clozapine for the resistant schizophrenics with agitation and aggressive behaviour. Medical Journal of Chinese People's Health 2006;18(6):427‐8.
Garver 1983 {published data only}
-
- Garver DL, Hirschowitz J, Fleishmann R, Djuric P. Lithium response and psychoses: a double‐blind, placebo‐controlled study. Psychiatry Research 1984 May;12:57‐68. - PubMed
He 2010 {published data only}
-
- He Y‐F, Xie W‐C, Li X‐Y. Risperidone combined with lithium in treatment of refractory schizophrenia. Guangzhou Medical Journal 2010;2:6‐8.
Hogarty 1995 {published data only}
-
- Hogarty GE, McEvoy JP, Ulrich RF, DiBarry AL, Bartone P, Cooley S, Hammill K, Carter M, Munetz MR, Perel J. Pharmacotherapy of impaired affect in recovering schizophrenic patients. Archives of General Psychiatry 1995;52:29‐41. - PubMed
Huang 1984 {published data only}
-
- Huang LG BC. Platelet monoamine oxidase response to lithium treatment in psychiatric patients. Journal of Clinical Psychopharmacology 1984;4:326‐31. - PubMed
Johnson 1971 {published data only}
-
- Johnson G. Differential response to lithium carbonate in manic depressive and schizo‐affective disorders. Diseases of the Nervous System 1970;31:613‐5. - PubMed
-
- Johnson G, Gershon S, Burdock EI, Floyd A, Hekimian L. Comparative effects of lithium and chlorpromazine in the treatment of acute manic states. British Journal of Psychiatry 1971;119:267‐76. - PubMed
-
- Johnson G, Gershon S, Hekimian L. Controlled evaluation of lithium and chlorpromazine in the treatment of manic states: an interim report. Comprehensive Psychiatry 1968;9:563‐73. - PubMed
Johnstone 1988 {published data only}
-
- Johnstone EC, Crow TJ, Frith CD, Owens D. The Northwick Park "functional" psychosis study: diagnosis and treatment response. Lancet 1988;2:119‐25. - PubMed
-
- Johnstone EC, Crow TJ, Owens D, Frith C. The Northwick Park 'Functional' Psychosis Study. Phase 2 ‐ Maintenance treatment. Journal of Psychopharmacology 1991;5:388‐95. - PubMed
-
- Johnstone EC, Owens DGC. Does early treatment have an effect on outcome?. 10th ECNP (European College of Neuropsychopharmacology) Congress, Vienna, Austria. Sep 13 ‐ 17, 1997.
-
- Johnstone EC, Owens DGC, Crow TJ, Davis JM. Does a four week delay in the introduction of medication alter the course of functional psychosis?. Journal of Psychopharmacology 1999; Vol. 13, issue 3:238‐44. [MEDLINE: ] - PubMed
Mattes 1984 {published data only}
-
- Mattes JA, Nayak D. Lithium versus fluphenazine for prophylaxis in mainly schizophrenic schizoaffectives. Biological Psychiatry 1984;19:445‐9. - PubMed
Prien 1972 {published data only}
-
- Pokorny AD, Prien RF. Lithium in treatment and prevention of affective disorder: A VA NIMH collaborative study. Diseases of the Nervous System 1974;35:327‐33. - PubMed
-
- Prien R, Caffey EJ, Klett C. A comparison of lithium carbonate and chlorpromazine in the treatment of excited schizo‐affectives. Report of the Veterans Administration and National Institute of Mental Health collaborative study group. Archives of General Psychiatry 1972;27:182‐9. - PubMed
Schulz 1999 {published data only}
-
- Schultz SC, Glick D, Group TSSCS. Lithium augmentation fails to reduce symptoms in poorly responsive schizophrenic outpatients. Schizophrenia Research 1995; Vol. 15, issue 1‐2:151. [MEDLINE: ] - PubMed
-
- Schulz SC, Thompson PA, Jacobs M, Ninan PT, Robinson D, Weiden PJ, Yadalam K, Glick ID, Odbert CL. Lithium augmentation fails to reduce symptoms in poorly responsive schizophrenic outpatients. Journal of Clinical Psychiatry 1999;60:366‐72. - PubMed
Shopsin 1971 {published data only}
-
- Shopsin B, Kim S, Gershon S. A controlled study of lithium vs. chlorpromazine in acute schizophrenics. British Journal of Psychiatry 1971;119:435‐40. - PubMed
Simhandl 1996 {published data only}
-
- Simhandl C, Meszaros K, Denk E, Thau K, Topitz A. Adjunctive carbamazepine or lithium carbonate in therapyresistant chronic schizophrenia [2]. Canadian Journal of Psychiatry/ Revue Canadienne de Psychiatrie 1996;41(5):317. - PubMed
Simpson 1976 {published data only}
-
- Simpson GM, Branchey MH, Lee JH, Voitashevsky A, Zoubok B. Lithium in tardive dyskinesia. Pharmakopsychiatrie‐Neuropsychopharmakologie 1976;9:76‐80. - PubMed
Small 1975 {published data only}
-
- Kellams J, Small J, Milstein V, Perex H. Lithium combined with neuroleptics in the treatment of chronic schizophrenia. Psychopharmacology Bulletin 1976;12:27‐30. - PubMed
-
- Schulz SC, Kahn EM, Baker RW, Conley RR. Lithium and carbamazepine augmentation in treatment‐refractory schizophrenia. The Neuroleptic‐Nonresponsive Patient: Characterization and Treatment. Washington, DC, USA: American Psychiatric Press Inc, 1990:111‐36. [MEDLINE: ]
-
- Small JG, Kellams JJ, Milstein V, Moore J. A placebo‐controlled study of lithium combined with neuroleptics in chronic schizophrenic patients. American Journal of Psychiatry 1975;132:1315‐7. - PubMed
Small 2001 {published and unpublished data}
-
- Small JG, Klapper MH, Malloy FW, Steadman TM. Clozapine combined with lithium in schizophrenia and schizoaffective disorder. Schizophrenia Research 2001;49 (suppl):246. - PubMed
-
- Small JG, Klapper MH, Malloy FW, Steadman TM. Tolerability and efficacy of clozapine combined with lithium in schizophrenia and schizoaffective disorder. Journal of clinical psychopharmacology. United States of America, 2003; Vol. 23, issue 3:223‐8. [MEDLINE: ] - PubMed
Terao 1995 {published data only}
-
- Terao T, Oga T, Nozaki S, Ohta A, Ohtsubo Y, Yamamoto S, Zamami M, Okada M. Lithium addition to neuroleptic treatment in chronic schizophrenia: a randomized, double‐blind, placebo‐controlled, cross‐over study. Acta Psychiatrica Scandinavica 1995;92:220‐4. - PubMed
-
- Terao T, Oga T, Nozaki S, Ohta A, Ohtsubo Y, Yamoto S, et al. L'associazione di Litio alla terapia neurolettica nel trattamento della schizofrenia cronica: uno studio in doppio cieco crociato randomizzato, controllato con placebo. Rivista di Psichiatria. Italy: Il Pensiero Scientifico Editore, 1995; Vol. 30, issue 6:42. [MEDLINE: ]
Wilson 1993 {published data only}
-
- Wilson WH. Addition of lithium to haloperidol in non ‐ affective, antipsychotic non ‐ responsive schizophrenia. Schizophrenia Research 1993; Vol. 9:256. [MEDLINE: ] - PubMed
-
- Wilson WH. Addition of lithium to haloperidol in non‐affective, antipsychotic non‐responsive schizophrenia ‐ A double blind, placebo controlled, parallel design clinical trial. Psychopharmacology 1993;111:359‐66. - PubMed
References to studies excluded from this review
Alexander 1979 {published data only}
-
- Alexander PE, Kammen DP, Bunney WE. Antipsychotic effects of lithium in schizophrenia. American Journal of Psychiatry 1979;136:283‐7. - PubMed
Aronoff 1970 {published data only}
-
- Aronoff MS, Epstein RS. Factors associated with poor response to lithium carbonate: a clinical study. American journal of psychiatry 1970; Vol. 127:472‐80. [MEDLINE: ] - PubMed
Baastrup 1967 {published data only}
-
- Baastrup PC, Schou G, Schou M. Lithium as a prophylactic agent. Archives of General Psychiatry 1967;16:162‐72. - PubMed
Bellaire 1990 {published data only}
-
- Bellaire W, Demisch K, Stoll KD. Carbamazepine versus lithium. Application in the prophylaxis of recidivating affective and schizoaffective psychoses [Carbamazepin Vs. Lithium. Einsatz in der Prophylaxe Rezidivierender Affektiver und Schizoaffektiver Psychosen]. Munchener Medizinische Wochenschriftenschrift 1990; Vol. 132:82‐6. [MEDLINE: ]
Berger 2006 {published data only}
-
- Berger G. Lithium in patients at ultra high risk of developing a first psychotic episode. Stanley Foundation Research Programs 2006.
Bigelow 1981 {published data only}
-
- Bigelow LB, Weinberger DR, Wyatt R. Synergism of combined lithium‐neuroleptic therapy ‐ A double‐blind, placebo‐controlled case study. American Journal of Psychiatry 1981;138:81‐3. - PubMed
Bowers 1983 {published data only}
-
- Bowers MB, Heninger GR, Sternberg DE. Correlates of lithium response in psychotic‐affective syndromes. Comprehensive psychiatry 1983;24:469‐75. - PubMed
Braden 1980 {published data only}
-
- Braden W. Lithium versus chlorpromazine in acute psychosis. Psychopharmacology Bulletin 1980; Vol. 16:70‐1. [MEDLINE: ]
Brewerton 1983 {published data only}
-
- Brewerton TD, Reus VI. Lithium carbonate and l‐tryptophan in the treatment of bipolar and schizoaffective disorders. American journal of psychiatry 1983; Vol. 140:757‐60. [MEDLINE: ] - PubMed
Cabrera 1986 {published data only}
-
- Cabrera JF, Muhlbauer HD, Schley J, Stoll KD, Muller‐Oerlinghausen B. Long‐term randomized clinical trial on oxcarbazepine vs lithium in bipolar and schizoaffective disorders ‐ Preliminary results. Pharmacopsychiatry 1986; Vol. 19, issue 4:282‐3. [MEDLINE: ]
Campbell 1972 {published data only}
-
- Campbell M, Fish B, Korein J, Shapiro T, Collins P, Koh C. Lithium and chlorpromazine: a controlled cross‐over study of hyperactive severely disturbed young children. Journal of Autism and Childhood Schizophrenia 1972;2:234‐63. - PubMed
Campbell 1982 {published data only}
-
- Campbell M, Cohen IL, Small AM. Drugs in aggressive behavior. Journal of the American Academy of Child Psychiatry 1982; Vol. 21, issue 2:107‐17. [MEDLINE: ] - PubMed
Carman 1981 {published data only}
-
- Carman J, Bigelow LB, Wyatt RJ. Lithium combined with neuroleptics in chronic schizophrenic and schizoaffective patients. Journal of Clinical Psychiatry 1981;42:124‐8. - PubMed
Chen 2001 {published data only}
-
- Chen L, Zhang X, Li J. A controlled study between clozapine and lithium carbonate in treatment‐refractory schizophrenia. Sichuan Mental Health 2001;14:213‐4.
Dinsmore 1972 {published data only}
-
- Dinsmore PR, Ryback R. Lithium in schizo‐affective disorders. Diseases of the Nervous System 1972;33:771‐6. - PubMed
Edelstein 1981 {published data only}
-
- Edelstein P, Schultz JR, Hirschowitz J, Kanter DR, Garver D. Physostigmine and lithium response in the schizophrenias. American Journal of Psychiatry 1981;138:1078‐81. - PubMed
Gao 2002 {published data only}
-
- Gao S, Ma J, Xu F, Yang S. Clozapine combined with lithium carbonate treatment for patients with chronic schizophrenia: controlled clinical trial. Nervous Diseases and Mental Hygiene 2002;2:338‐9.
Garver 1988 {published data only}
-
- Garver DL, Kelly K, Fried KA, Magnusson M, Hirschowitz J. Drug response patterns as a basis of nosology for the mood‐incongruent psychoses (the schizophrenias). Psychological Medicine 1988;18:873‐85. - PubMed
Gerlach 1975 {published data only}
-
- Gerlach J, Thorsen K, Munkvad I. Effect of lithium on neuroleptic‐induced tardive dyskinesia compared with placebo in a double‐blind cross‐over trial. Pharmakopsychiatrie Neuropsychopharmakologie 1975;8:51‐6. - PubMed
Gram 1971 {published data only}
-
- Gram LF, Rafaelsen OJ. Lithium treatment of psychotic children and adolescents. A controlled clinical trial. Acta Psychiatrica Scandinavica 1972;48:253‐60. - PubMed
Greil 1997 {published data only}
-
- Greil W, Ludwig Mayerhofer W, Erazo N, Engel RR. Lithium vs cambamazepine in the maintenance treatment of schizoaffective disorder: A randomised study. European Archives of Psychiatry and Clinical Neuroscience 1997;247:42‐50. - PubMed
Growe 1979 {published data only}
-
- Growe GA, Crayton JW, Klass DB, Evans H, Strizich M. Lithium in chronic schizophrenia. American Journal of Psychiatry 1979;136:454‐5. - PubMed
Haastrup 1973 {published data only}
-
- Haastrup S, Rasmussen S, Kirk L. Lithium therapy of schizophrenics. A controlled study. Acta Psychiatrica Scandinavica Supplement 1973;243:68.
Harrison 1980 {published data only}
-
- Harrison GP, Lipinski JF, Cohen BM, Aselrod DT. "Schizoaffective disorder": an invalid diagnosis? A comparison of schizoaffective disorder, schizophrenia, and affective disorder. American Journal of Psychiatry 1980;137:921‐7. - PubMed
Hofmann 1970 {published data only}
-
- Hofmann G, Kremser M, Katschnig H, Scheiber V. Prophylaktische Lithiumtherapie bei manisch‐depressivem Krankheitsgeschehen und bei Legierungspsychosen. Internationale Pharmacopsychiatrie 1970;4:187‐93.
Hullin 1975 {published data only}
-
- Hullin RP, McDonald R, Allsopp MNE. Further report on prophylactic lithium in recurrent affective disorders. British Journal of Psychiatry 1975;126:281‐4. - PubMed
Jus 1978 {published data only}
-
- Jus A, Villeneuve A, Gautier J, Jus K, Villeneuve C, Pires P, Villeneuve R. Deanol, lithium and placebo in the treatment of tardive dyskinesia. A double‐blind crossover study. Neuropsychobiology 1978;4:140‐9. - PubMed
Kahn 1990 {published data only}
-
- Kahn EM, Schulz SC, Perel JM, Alexander JE. Change in haloperidol level due to carbamazepine ‐ a complicating factor in combined medication for schizophrenia. Journal of clinical psychopharmacology 1990; Vol. 10, issue 1:54‐7. [MEDLINE: ] - PubMed
Lenzi 1985 {published data only}
-
- Lenzi A, Lazzerini F, Grossi E. Use of carbamazepine in acute psychosis ‐ A controlled study. Journal of International Medical Research 1986;14:78‐84. - PubMed
Lerner 1988 {published data only}
-
- Lerner Y, Mintzer Y, Schestatzky M. Lithium combined with haloperidol in schizophrenic patients. British Journal of Psychiatry 1988;153:359‐62. - PubMed
Liebowitz 1976 {published data only}
-
- Liebowitz JH, Rudy V, Gershon ES, Gillis A. A pharmacogenetic case report: Lithium‐responsive postpsychotic antisocial behavior. Comprehensive Psychiatry 1976;17:655‐60. - PubMed
Liu 2006 {published data only}
-
- Liu N. The lithium compound norethisterone periodic relapse prevention control study [碳酸锂与复方炔诺酮预防周期性精神病复发的对照研究]. Medical Journal of Chinese People's Health [中国民康医学] 2006; Vol. 18, issue 5:199‐200.
Mackkay 1980 {published data only}
-
- MacKay AV, Sheppard GP, Saha BK, Motley B, Johnson AL, Marsden CD. Failure of lithium treatment in established tardive dyskinesia. Psychological Medicine 1980;10:583‐7. - PubMed
Marken 1994 {published data only}
-
- Marken PA, McCrary KE, Lacombe S, Sommi RW, Hornstra RK, Pierce CA, et al. Preliminary comparison of predictive and empiric lithium dosing: impact on patient outcome. Annals of Pharmacotherapy 1994; Vol. 28, issue 10:1148‐52. [MEDLINE: ] - PubMed
Martorano JT {published data only}
-
- Martorano JT. Target symptoms in lithium carbonate therapy. Comprehensive Psychiatry 1972;13:533‐7. - PubMed
Miller 1979 {published data only}
-
- Miller V. Lithium carbonate in the treatment of schizophrenia and schizo‐affective disorder: Review and hypothesis. Journal of Clinical Psychiatry 1979;14:705‐10. - PubMed
Nct 2005 {published data only}
-
- Nct. Divalproex extended release and placebo, lithium, or quetiapine for mania. http://www.clinicaltrials.gov 2005.
Nemes 1986 {published data only}
-
- Nemes ZC, Volavka J, Cooper TB, O'Donnell J, Jaeger J. Lithium and haloperidol. Biological Psychiatry 1986;21:568‐9. - PubMed
Paykel 2000 {published data only}
-
- Paykel ES. Prophylaxis of puerperal psychosis. National Research Register 2000.
Placidi 1986 {published data only}
-
- Placidi GF, Lenzi A, Lazzerini F, Cassano G. The comparative efficacy and safety of carbamazepine versus lithium: A randomized, double blind 3 year trial in 83 patients. Journal of Clinical Psychiatry 1986;47:490‐4. - PubMed
-
- Placidi GF, Lenzi A, Rampello E, Andreani MF, Cassano GB, Grossi E. Long term‐double blind prospective study on carbamazepine versus lithium in bipolar and schizoaffective disorders. Preliminary results. Anticonvulsants in Affective Disorders. Amsterdam, Netherlands: Elsevier, 1984:188‐97. [MEDLINE: ]
Prange 1973 {published data only}
-
- Prange A. Preliminary experience with tryptophan and lithium in the treatment of tardive dyskinesia. Psychopharmacology Bulletin 1973;9:36‐7.
Prien 1972a {published data only}
-
- Prien RF, Caffey EM, Klett J. Factors associated with treatment in lithium carbonate prophylaxis. Archives of General Psychiatry 1974;31:189‐92. - PubMed
-
- Prien RF, Point P, Caffey EM, Klett CJ. Comparison of lithium carbonate and chlorpromazine in the treatment of mania. Archives of General Psychiatry 1972;26:146‐53. - PubMed
Rice 1956 {published data only}
-
- Rice D. The use of lithium salts in the treatment of manic states. International Pharmacology 1956;3:604‐11. - PubMed
Rosenthal 1980 {published data only}
-
- Rosenthal NE, Rosenthal LN, Stallone F, Dunner DL, Fieve RR. Toward the validation of RDC schizoaffective disorder. Archives of General Psychiatry 1980;37:804‐10. - PubMed
Schnexnayder 1995 {published data only}
-
- Schnexnayder LW, Hirschowitz J, Sautter FJ, Garver DL. Predictors of response to lithium in patients with psychoses. American Journal of Psychiatry 1995;152:1511‐3. - PubMed
Schou 1954 {published data only}
Shalif 1981 {published data only}
-
- Shalif I, Lerner Y, Dasberg H. A symptom profile analysis of antipsychotic drug treatment ‐ nonparametric multidimensional technique. Psychiatry research 1981; Vol. 4, issue 1:1‐12. [MEDLINE: ] - PubMed
Shaw 1974 {published data only}
-
- Shaw DM, Hewland R, Johnson AL, Hilary‐Jones P, Howlett MR. Comparison of serum levels of two sustained‐release preparations of lithium carbonate. Current Medical Research Opinion 1974;2:90‐4. - PubMed
Shopsin 1975 {published data only}
-
- Shopsin B, Gershon S, Thompson H, Collins P. Psychoactive drugs in mania. Archives of General Psychiatry 1975;32:34‐42. - PubMed
Smulevitch 1974 {published data only}
-
- Smulevitch AB, Zavidovskaya GI, Igonin AL, Mikhailova NM. The effectiveness of lithium in affective and schizoaffective psychoses. British Journal of Psychiatry 1974;125:65‐72. - PubMed
Sun 2008 {published data only}
-
- Sun H, Jiao Y‐T, Zhang L‐X. A comparative study of clozapine and clozapine unite lithium carbonate in the treatment of male patients with treatment‐resistant schizophrenia. Journal of Sichuang Mental Health 2008;21(2):83‐5.
Taylor 1974 {published data only}
-
- Taylor MA, Abrams R. The phenomenology of mania. Archives of General Psychiatry 1973;29:520‐2. - PubMed
-
- Taylor MA, Gaztanaga P, Abrams R. Manic‐depressive illness and acute schizophrenia: a clinical, family history, and treatment‐response study. American Journal of Psychiatry 1974;131:678‐82. - PubMed
Van Kammen 1978 {published data only}
-
- Kammen DP, Alexander P. d Amphetamine raises serum prolactin in man: Evaluations after chronic placebo, lithium and pimozide treatment. Life Sciences 1978;23:1487‐92. - PubMed
Van Kammen 1980 {published data only}
-
- Kammen D, Alexander P, Bunney WJ. Lithium treatment in post‐psychotic depression. British Journal of Psychiatry 1980;136:479‐85. - PubMed
Van Kammen 1985 {published data only}
-
- Kammen DP, Docherty JP, Marder SR. Lithium attenuates the activation‐euphoria but not the psychosis induced by d‐amphetamine in schizophrenia. Psychopharmacology 1985;87:111‐5. - PubMed
Van Putten 1975 {published data only}
-
- Putten T, Sanders D. Lithium in treatment failures. Journal of Nervous and Mental disease 1975;161:255‐64. - PubMed
Vieweg 1989 {published data only}
-
- Vieweg V, Godleski L, Hundley P, Harrington D, Yank G. Antipsychotic drugs, lithium, carbamazepine, and abnormal diurnal weight gain in psychosis. Neuropsychopharmacology 1989;2:39‐43. - PubMed
Volavka 1986 {published data only}
-
- Volavka J, O'Donald J, Muragali R. Lithium and lecithin in tardive dyskinesia: An update. Psychiatry Research 1986;19:101‐4. - PubMed
Wang 1995 {published data only}
-
- Wang Q, Yuan F, Ai L, Yin D, Xiao Y. Chlorpromazine combined with lithium carbonate treatment for patients with refractory schizophrenia: a randomized double‐blind cross‐over clinical trial. Journal of Clinical Psychological Medicine 1995;5:185.
White 1966 {published data only}
-
- White RB, Schlagenhauf G, Tupin JP. The treatment of manic depressive states wtih lithium carbonate. Current Psychiatric Therapies 1966;11:230‐42.
Wilner 1996 {published data only}
Wilson 1994 {published data only}
-
- Wilson WH. Open clozapine treatment following a controlled clinical trial of lithium augmentation of haloperidol for refractory schizophrenia. Lithium 1994; Vol. 5, issue 2:113‐4.
Zemlan 1984 {published data only}
-
- Zemlan FP, Hirschowitz J, Sautter FJ, Garver DL. Impact of lithium therapy on core psychotic symptoms of schizophrenia. British Journal of Psychiatry 1984;144:64‐9. - PubMed
Zhou 2011 {published data only}
-
- Zhou Guangyu, Rong Min. Paroxetine combined with lithium in patients with refractory schizophrenia.. Medical Journal of Chinese People's Health 2011;23(15):1899‐1900.
References to studies awaiting assessment
Kamisada 1988 {published data only}
-
- Kamisada M, Tateyama M, Nakano Y, Kawachi Y, FUjii Y, Tanoue A, Takamiya M, Nakajima S, Sakuma K, Oguchi E, Yagi G. Comparison of the clinical effects of lithium carbonate and carbamazepine on excited schizophrenics. Psychopharmacology 1988;96 (suppl):348.
McGorry 2002 {published data only}
-
- McGorry PD, Cocks J, Harrigan S, Elkins K, Lambert T, Owen S. Very low‐dose risperidone treatment in first‐episode psychosis: how effective is it?. Proceedings of the 3rd International Conference on Early Psychosis; 2002 Sep 25‐28; Copenhagen, Denmark 2002:48.
Mosolov 1998 {published data only}
-
- Mosolov SN, Kostiukova EG, Kuzavkova MV. The efficacy of lithium carbonate and contemnol in preventing affective and schizoaffective psychoses. Zhurnal Nevropatologii i Psikhiatrii Imeni S. S. Korsakova 1998; Vol. 98, issue 3:15‐8. [MEDLINE: ] - PubMed
Additional references
Altman 1996
Andreasen 1989
-
- Andreasen NC. Scale for the assessment of negative symptoms. British Journal of Psychiatry 1989;155:53‐8. - PubMed
Astrachan 1972
-
- Astrachan BM, Harrow M, Adler D, Brauer L, Schwartz A, Tucker G. A scale for the diagnosis of schizophrenia. British Journal of Psychiatry 1972;121:529. - PubMed
Atre‐Vaidya 1989
-
- Atre‐Vaidya N, Taylor MA. Effectiveness of lithium in schizophrenia: Do we really have an answer?. Journal of Clinical Psychiatry 1989;50(5):170‐173. - PubMed
Barnes 1989
-
- Barnes TRE. A rating scale for drug‐induced akathisia. British Journal of Medicine 1989;154:672‐6. - PubMed
Bech 1978
-
- Bech P, Rafaelsen OJ, Kramp P, Bolwig TG. The mania rating scale: scale construction and inter‐observer agreement. Neuropharmacology 1978;17:430‐1. - PubMed
Bland 1997
Boissel 1999
-
- Boissel JP, Cucherat M, Li W, Chatellier G, Gueyffier F, Buyse M, et al. The problem of therapeutic efficacy indices. 3. Comparison of the indices and their use [Apercu sur la problematique des indices d'efficacite therapeutique, 3: comparaison des indices et utilisation. Groupe d'Etude des Indices D'efficacite]. Therapie 1999;54(4):405‐11. [PUBMED: 10667106] - PubMed
Burdock 1968
-
- Burdock EI, Hardesty, AS. A psychological test for psychopathology. Journal of Abnormal Psychology 1968;73:62‐9. - PubMed
Carpenter 1994
-
- Carpenter WT Jr, Buchanan RW. Schizophrenia. New England Journal of Medicine 1994;330:681‐90. - PubMed
Cheine 2001
-
- Cheine M, Ahonen J, Wahlbeck K. Supplementing standard drug treatment of those with schizophrenia with beta‐blocking medication. Cochrane Database of Systematic Reviews 2001, Issue 3. [DOI: 10.1002/14651858.CD000234] - DOI
Christison 1991
-
- Christison GW, Kirch DG, Wyatt RJ. When symptoms persist: choosing among alternative somatic treatments for schizophrenia. Schizophrenia Bulletin 1991;17:217‐45. - PubMed
Citrome 2009
-
- Citrome L. Adjunctive lithium and anticonvulsants for the treatment of schizophrenia: what is the evidence?. Expert Rev Neurother 2009;9(1):55‐71. - PubMed
Davey Smith 1998
Deeks 2000
-
- Deeks J. Issues in the selection for meta‐analyses of binary data. Abstracts of 8th International Cochrane Colloquium; 2000 Oct 25‐28th; Cape Town, South Africa. 2000.
Delva 1982
-
- Delva, NJ, Letemendia. FJ. Lithium treatment in schizophrenia and schizo‐affective disorders. The British Journal of Psychiatry 1982;141:387‐400. - PubMed
Divine 1992
-
- Divine GW, Brown JT, Frazer LM. The unit of analysis error in studies about physicians' patient care behavior. Journal of General Internal Medicine 1992;7:623‐9. - PubMed
Dold 2012
Donner 2002
-
- Donner A, Klar N. Issues in the meta‐analysis of cluster randomized trials. Statistics in Medicine 2002;21:2971‐80. - PubMed
Egger 1997
Elbourne 2002
-
- Elbourne D, Altman DG, Higgins JPT, Curtina F, Worthingtond HV, Vaile A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. - PubMed
Essali 2009
Furukawa 2006
-
- Furukawa TA, Barbui C, Cipriani A, Brambilla P, Watanabe N. Imputing missing standard deviations in meta‐analyses can provide accurate results. Journal of Clinical Epidemiology 2006;59(7):7‐10. - PubMed
GRADE Profiler [Computer program]
-
- GRADE Working Group. GRADE Profiler. Version 3.2. GRADE Working Group, 2004.
Gulliford 1999
-
- Gulliford MC, Ukoumunne OC, Chinn S. Components of variance and intraclass correlations for the design of community‐based surveys and intervention studies: data from the Health Survey for England 1994. American Journal of Epidemiology 1999;149:876‐83. - PubMed
Guy 1976
-
- Guy U. ECDEU assessment manual for psychopharmacology. Revised. Rockville, Maryland, USA: National Institute of Mental Health, 1976.
Hamilton 1960
Higgins 2003
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. Available from www.cochrane‐handbook.org. [updated March 2011].
Jope 1999
-
- Jope RS. Anti‐bipolar therapy: mechanism of action of lithium. Molecular Psychiatry 1999;4(2):117‐28. - PubMed
Kay 1986
-
- Kay SR, Opler LA, Fiszbein A. Positive and negative syndrome scale (PANSS) manual. Positive and negative syndrome scale (PANSS) manual. North Tonawanda, NY: Multi‐Health Systems, 1986.
Kay 1987
-
- Kay SR, Fiszbein A. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophrenia Bulletin 1987;13(2):261‐75. - PubMed
Krawiecka 1977
-
- Krawiecka M, Goldberg D, Vaughan MA. Standardized assessment scale for rating chronic psychotic patients. Acta Psychiatrica Scandinavica 1977;55:299‐308. - PubMed
Lenox 2003
-
- Lenox RH, Wang L. Molecular basis of lithium action: integration of lithium‐responsive signaling and gene expression networks. Molecular Psychiatry 2003;8(2):135‐44. - PubMed
Leucht 2005
-
- Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel R. Clinical implications of Brief Psychiatric Rating Scale scores. British Journal of Psychiatry 2005;187:366‐71. [PUBMED: 16199797] - PubMed
Leucht 2005a
-
- Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel RR. What does the PANSS mean?. Schizophrenia Research 2005;79(2‐3):231‐8. [PUBMED: 15982856] - PubMed
Leucht 2013
-
- Leucht S, Cipriani A, Spineli L, Mavridis D, Orey D, Richter F, Samara M, Barbui C, Engel RR, Geddes JR, Kissling W, Stapf MP, Lässig B, Salanti G, Davis JM. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple‐treatments meta‐analysis. The Lancet 2013;382(9896):951‐962. - PubMed
Leucht 2014
Marshall 2000
-
- Marshall M, Lockwood A, Bradley C, Adams C, Joy C, Fenton M. Unpublished rating scales: a major source of bias in randomised controlled trials of treatments for schizophrenia. British Journal of Psychiatry 2000;176:249‐52. - PubMed
Marvaha 2004
-
- Marwaha S, Johnson S. Schizophrenia and employment ? A review.. Social Psychiatry and Psychiatric Epidemiology 2004;39:337‐49. - PubMed
Montgomery 1979
-
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. British Journal of Psychiatry 1979;134:382‐9. - PubMed
NIMH 1970
-
- National Institute of Mental Health. Clinical global impression. In: Guy W, Bonato RR editor(s). Manual for the ECDEU Assessment Battery. Washington DC: NIMH, 1970.
Overall 1962
-
- Overall JE, Gorham DR. The brief psychiatric rating scale. Psychological Reports 1962;10:790‐812.
Review Manager (RevMan) [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.0. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2008.
Schooler 1993
-
- Schooler NR, Keith SJ. Clinical research for the treatment of schizophrenia. Psychopharmacology Bulletin 1993;29:431‐46. - PubMed
Schulz 1995
-
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias: dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273:408‐12. - PubMed
Schulz 2010
Schwarz 2008
Schünemann 2008
-
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration, 2008:359‐83.
Simpson 1970
-
- Simpson M, Angus JW. A rating scale for extrapyramidal side effects. Acta Psychiatrica Scandinavica 1970;212:11‐19. - PubMed
SPSS 2001 [Computer program]
-
- SPSS Inc. SPSS for Windows version 11.0.1. LEEDS technologies Inc., 2001.
Tharyan 2005
Tsuang 1978
-
- Tsuang MT. Suicide in schizophrenics, manics, depressives, and surgical controls: a comparison with general population suicide mortality.. Archives of General Psychiatry 1978;35:153‐55. - PubMed
Ukoumunne 1999
-
- Ukoumunne OC, Gulliford MC, Chinn S, Sterne JAC, Burney PGJ. Methods for evaluating area‐wide and organistation‐based intervention in health and health care: a systematic review. Health Technology Assessment 1999;3(5):1‐75. - PubMed
Wahlbeck 2001
-
- Wahlbeck K, Tuunainen A, Ahokas A, Leucht S. Drop‐out rates in randomised antipsychotic drug trials. Psychopharmacology 2001;155:230‐3. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

