In vivo evidence for an endothelium-dependent mechanism in radiation-induced normal tissue injury
- PMID: 26510580
- PMCID: PMC4625166
- DOI: 10.1038/srep15738
In vivo evidence for an endothelium-dependent mechanism in radiation-induced normal tissue injury
Abstract
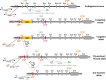
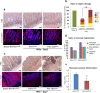
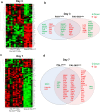
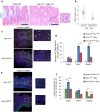
The pathophysiological mechanism involved in side effects of radiation therapy, and especially the role of the endothelium remains unclear. Previous results showed that plasminogen activator inhibitor-type 1 (PAI-1) contributes to radiation-induced intestinal injury and suggested that this role could be driven by an endothelium-dependent mechanism. We investigated whether endothelial-specific PAI-1 deletion could affect radiation-induced intestinal injury. We created a mouse model with a specific deletion of PAI-1 in the endothelium (PAI-1KO(endo)) by a Cre-LoxP system. In a model of radiation enteropathy, survival and intestinal radiation injury were followed as well as intestinal gene transcriptional profile and inflammatory cells intestinal infiltration. Irradiated PAI-1KO(endo) mice exhibited increased survival, reduced acute enteritis severity and attenuated late fibrosis compared with irradiated PAI-1(flx/flx) mice. Double E-cadherin/TUNEL labeling confirmed a reduced epithelial cell apoptosis in irradiated PAI-1KO(endo). High-throughput gene expression combined with bioinformatic analyses revealed a putative involvement of macrophages. We observed a decrease in CD68(+)cells in irradiated intestinal tissues from PAI-1KO(endo) mice as well as modifications associated with M1/M2 polarization. This work shows that PAI-1 plays a role in radiation-induced intestinal injury by an endothelium-dependent mechanism and demonstrates in vivo that the endothelium is directly involved in the progression of radiation-induced enteritis.
Figures



References
-
- Bentzen S. M. Preventing or reducing late side effects of radiation therapy: radiobiology meets molecular pathology. Nat Rev Cancer 6, 702–713 (2006). - PubMed
-
- Andreyev H. J., Wotherspoon A., Denham J. W. & Hauer-Jensen M. Defining pelvic-radiation disease for the survivorship era. Lancet Oncol 11, 310–312 (2010). - PubMed
-
- Baker D. G. & Krochak R. J. The response of the microvascular system to radiation: a review. Cancer Invest 7, 287–294 (1989). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

