Innervation of the rabbit cardiac ventricles
- PMID: 26510903
- PMCID: PMC4694158
- DOI: 10.1111/joa.12400
Innervation of the rabbit cardiac ventricles
Abstract
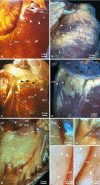
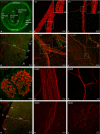
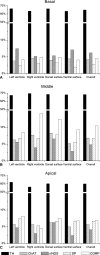
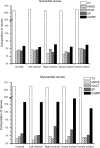
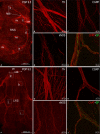
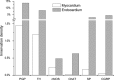
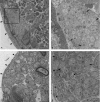
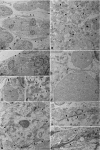
The rabbit is widely used in experimental cardiac physiology, but the neuroanatomy of the rabbit heart remains insufficiently examined. This study aimed to ascertain the architecture of the intrinsic nerve plexus in the walls and septum of rabbit cardiac ventricles. In 51 rabbit hearts, a combined approach involving: (i) histochemical acetylcholinesterase staining of intrinsic neural structures in total cardiac ventricles; (ii) immunofluorescent labelling of intrinsic nerves, nerve fibres (NFs) and neuronal somata (NS); and (iii) transmission electron microscopy of intrinsic ventricular nerves and NFs was used. Mediastinal nerves access the ventral and lateral surfaces of both ventricles at a restricted site between the root of the ascending aorta and the pulmonary trunk. The dorsal surface of both ventricles is supplied by several epicardial nerves extending from the left dorsal ganglionated nerve subplexus on the dorsal left atrium. Ventral accessing nerves are thicker and more numerous than dorsal nerves. Intrinsic ventricular NS are rare on the conus arteriosus and the root of the pulmonary trunk. The number of ventricular NS ranged from 11 to 220 per heart. Four chemical phenotypes of NS within ventricular ganglia were identified, i.e. ganglionic cells positive for choline acetyltransferase (ChAT), neuronal nitric oxide synthase (nNOS), and biphenotypic, i.e. positive for both ChAT/nNOS and for ChAT/tyrosine hydroxylase. Clusters of small intensely fluorescent cells are distributed within or close to ganglia on the root of the pulmonary trunk, but not on the conus arteriosus. The largest and most numerous intrinsic nerves proceed within the epicardium. Scarce nerves were found near myocardial blood vessels, but the myocardium contained only a scarce meshwork of NFs. In the endocardium, large numbers of thin nerves and NFs proceed along the bundle of His and both its branches up to the apex of the ventricles. The endocardial meshwork of fine NFs was approximately eight times denser than the myocardial meshwork. Adrenergic NFs predominate considerably in all layers of the ventricular walls and septum, whereas NFs of other neurochemical phenotypes were in the minority and their amount differed between the epicardium, myocardium and endocardium. The densities of NFs positive for nNOS and ChAT were similar in the epicardium and endocardium, but NFs positive for nNOS in the myocardium were eight times more abundant than NFs positive for ChAT. Potentially sensory NFs positive for both calcitonin gene-related peptide and substance P were sparse in the myocardial layer, but numerous in epicardial nerves and particularly abundant within the endocardium. Electron microscopic observations demonstrate that intrinsic ventricular nerves have a distinctive morphology, which may be attributed to remodelling of the peripheral nerves after their access into the ventricular wall. In conclusion, the rabbit ventricles display complex structural organization of intrinsic ventricular nerves, NFs and ganglionic cells. The results provide a basic anatomical background for further functional analysis of the intrinsic nervous system in the cardiac ventricles.
Keywords: CGRP; PGP 9.5; SP; choline acetyltransferase; electron microscopy; heart ventricles; innervation; nNOS; rabbit; tyrosine hydroxylase.
© 2015 Anatomical Society.
Figures



References
-
- Anderson RH (1972b) Histologic and histochemical evidence concerning the presence of morphologically distinct cellular zones within the rabbit atrioventricular node. Anat Rec 173, 7–24. - PubMed
-
- Armour JA (2010) Functional anatomy of intrathoracic neurons innervating the atria and ventricles. Heart Rhythm 7, 994–996. - PubMed
-
- Armour JA, Murphy DA, Yuan BX, et al. (1997) Gross and microscopic anatomy of the human intrinsic cardiac nervous system. Anat Rec 247, 289–298. - PubMed
-
- Atkinson CJ, Santer RM (1999) Quantitative studies on myelinated and unmyelinated nerve fibres in the interatrial septal region of aged rat hearts. J Auton Nerv Syst 77, 172–176. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

