Generation of a miniature pig disease model for human Laron syndrome
- PMID: 26511035
- PMCID: PMC4625145
- DOI: 10.1038/srep15603
Generation of a miniature pig disease model for human Laron syndrome
Abstract
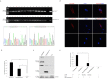
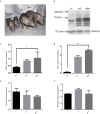
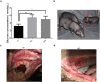
Laron syndrome is a rare disease caused by mutations of the growth hormone receptor (GHR), inheriting in an autosomal manner. To better understand the pathogenesis and to develop therapeutics, we generated a miniature pig model for this disease by employing ZFNs to knock out GHR gene. Three types of F0 heterozygous pigs (GHR(+/4bp), GHR(+/2bp), GHR(+/3bp)) were obtained and in which no significant phenotypes of Laron syndrome were observed. Prior to breed heterozygous pigs to homozygosity (GHR(4bp/4bp)), pig GHR transcript with the 4 bp insert was evaluated in vitro and was found to localize to the cytoplasm rather than the membrane. Moreover, this mutated transcript lost most of its signal transduction capability, although it could bind bGH. GHR(4bp/4bp) pigs showed a small body size and reduced body weight. Biochemically, these pigs exhibited significantly elevated levels of GH and decreased levels of IGF-I. These results resemble the phenotype observed in Laron patients, suggesting that these pigs could serve as an ideal model for Laron syndrome to bridge the gaps between mouse model and human.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



References
-
- Laron Z., Pertzelan A. & Mannheimer S. Genetic pituitary dwarfism with high serum concentation of growth hormone—a new inborn error of metabolism? Isr J Med Sci 2, 152–155 (1966). - PubMed
-
- Keret R., Pertzelan A., Zeharia A., Zadik Z. & Laron Z. Growth hormone (hGH) secretion and turnover in three patients with Laron-type dwarfism. Isr J Med Sci 24, 75–79 (1988). - PubMed
-
- Laron Z. et al.. Laron syndrome due to a post-receptor defect: response to IGF-I treatment. Isr J Med Sci 29, 757–763 (1993). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

