Voltage-Mediated Control of Spontaneous Bundle Oscillations in Saccular Hair Cells
- PMID: 26511238
- PMCID: PMC4623225
- DOI: 10.1523/JNEUROSCI.1451-15.2015
Voltage-Mediated Control of Spontaneous Bundle Oscillations in Saccular Hair Cells
Abstract
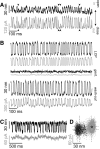
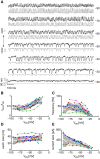
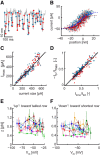
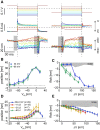
Hair cells of the vertebrate vestibular and auditory systems convert mechanical inputs into electrical signals that are relayed to the brain. This transduction involves mechanically gated ion channels that open following the deflection of mechanoreceptive hair bundles that reside on top of these cells. The mechano-electrical transduction includes one or more active feedback mechanisms to keep the mechanically gated ion channels in their most sensitive operating range. Coupling between the gating of the mechanosensitive ion channels and this adaptation mechanism leads to the occurrence of spontaneous limit-cycle oscillations, which indeed have been observed in vitro in hair cells from the frog sacculus and the turtle basilar papilla. We obtained simultaneous optical and electrophysiological recordings from bullfrog saccular hair cells with such spontaneously oscillating hair bundles. The spontaneous bundle oscillations allowed us to characterize several properties of mechano-electrical transduction without artificial loading the hair bundle with a mechanical stimulus probe. We show that the membrane potential of the hair cell can modulate or fully suppress innate oscillations, thus controlling the dynamic state of the bundle. We further demonstrate that this control is exerted by affecting the internal calcium concentration, which sets the resting open probability of the mechanosensitive channels. The auditory and vestibular systems could use the membrane potential of hair cells, possibly controlled via efferent innervation, to tune the dynamic states of the cells.
Keywords: frog; hair cell; sacculus; spontaneous oscillations.
Copyright © 2015 the authors 0270-6474/15/3514457-10$15.00/0.
Figures