Inflammation activation and resolution in human tendon disease
- PMID: 26511510
- PMCID: PMC4883654
- DOI: 10.1126/scitranslmed.aac4269
Inflammation activation and resolution in human tendon disease
Abstract
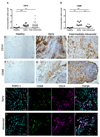
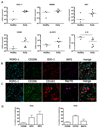
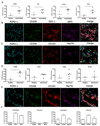
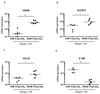
Improved understanding of the role of inflammation in tendon disease is required to facilitate therapeutic target discovery. We studied supraspinatus tendons from patients experiencing pain before and after surgical subacromial decompression treatment. Tendons were classified as having early, intermediate, or advanced disease, and inflammation was characterized through activation of pathways mediated by interferon (IFN), nuclear factor κB (NF-κB), glucocorticoid receptor, and signal transducer and activator of transcription 6 (STAT-6). Inflammation signatures revealed expression of genes and proteins induced by IFN and NF-κB in early-stage disease and genes and proteins induced by STAT-6 and glucocorticoid receptor activation in advanced-stage disease. The proresolving proteins FPR2/ALX and ChemR23 were increased in early-stage disease compared to intermediate- to advanced-stage disease. Patients who were pain-free after treatment had tendons with increased expression of CD206 and ALOX15 mRNA compared to tendons from patients who continued to experience pain after treatment, suggesting that these genes and their pathways may moderate tendon pain. Stromal cells from diseased tendons cultured in vitro showed increased expression of NF-κB and IFN target genes after treatment with lipopolysaccharide or IFNγ compared to stromal cells derived from healthy tendons. We identified 15-epi lipoxin A4, a stable lipoxin isoform derived from aspirin treatment, as potentially beneficial in the resolution of tendon inflammation.
Copyright © 2015, American Association for the Advancement of Science.
Conflict of interest statement
Figures



References
-
- Kannus P, Jozsa L. Histopathological changes preceding spontaneous rupture of a tendon. A controlled study of 891 patients. J Bone Joint Surg Am. 1991;73:1507–1525. - PubMed
-
- Judge A, Murphy RJ, Maxwell R, Arden NK, Carr AJ. Temporal trends and geographical variation in the use of subacromial decompression and rotator cuff repair of the shoulder in England. The bone & joint journal. 2014;96-B:70–74. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

