Allogeneic and Autogenous Bone Grafts Are Affected by Historical Donor Environmental Exposure
- PMID: 26511634
- PMCID: PMC4868162
- DOI: 10.1007/s11999-015-4572-7
Allogeneic and Autogenous Bone Grafts Are Affected by Historical Donor Environmental Exposure
Abstract
Background: Bone graft materials are routinely evaluated for infectious agents; however, data regarding contamination of bone graft from environmental exposure of the donors to osteotoxic substances such as lead are not routinely available. In animal models, stored lead in bone has been shown to impair fracture healing and osteocyte function. In clinical studies, lead is linked to skeletal disease at relatively low concentrations. Presumably the levels of lead in allografts mirror the level of lead in bone in the population; however, the degree to which processing might decrease this and the frequency with which potentially osteotoxic levels appear in bone grafts have not been studied.
Questions/purposes: (1) Does processing of donor bone for allografts result in lower concentrations of lead in commercial allograft when compared with autologous bone graft; and (2) what proportion of bone grafts contain potentially osteotoxic levels of lead from > 2.0 to 20.0 µg/g corresponding to environmental exposure?
Methods: Allograft from commercial sources and autologous bone graft materials were examined for lead content using ICP- atomic absorption spectrophotometric analysis. We analyzed bone graft specimens from 42 donors, including 26 corticocancellous tibial specimens from commercially available bone graft materials and 16 autograft corticocancellous tibial specimens. Lead levels were determined for the cortical (n = 42) and cancellous (n = 42) portions of each specimen. For quality control, all instruments, plastic and glassware, were regularly tested for lead contamination by atomic absorption spectrophotometry throughout the experiments. In addition, spectrophotometer calibration was verified using Standard Reference Material 1486 bone meal (NIST, Gaithersburg, MD, USA). Descriptive statistical analysis was performed using SPSS 20 (SPSS Inc, Chicago, IL, USA). Using these techniques, a lead level > 2 µg/g to 20 µg/g corresponds to some degree of environmental exposure to lead.
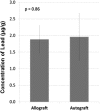
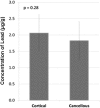
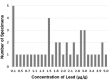
Results: With the numbers available in the present study, there were no differences in mean lead level between commercial bone graft materials and autogenous bone graft, 2.1 µg/g (95% confidence interval [CI], 1.6-3.3 µg/g) versus 2.0 µg/g (95% CI, 1.0-4.5 µg/g; p = 0.86). The range for all tested samples varied from < 0.1 to 5.0 µg/g. Likewise, there were no differences in mean lead level between cortical bone grafts, which contained 2.2 µg/g (95% CI, 1.5-3.7 µg/g), and cancellous grafts, which contained 1.9 µg/g (95% CI, 1.2-3.4 µg/g; p = 0.58). Thirty-eight percent (16 of 42) of the specimens had levels between 2.0 µg/g and 20 µg/g within a range expected for individuals with known environmental exposure to lead.
Conclusions: This study demonstrates that lead is present in up to one-third of tibial allograft and autograft bone specimens at potentially osteotoxic levels regardless of the source or screening. Further research is needed to delineate the relationship with nonunion or pseudoarthrosis after procedures in which allograft is used. In addition, further study would examine concentrations of lead and other environmental contaminants in other graft types.
Clinical relevance: Comparable levels of lead exposure have been associated with toxic effects on skeletal tissue. Further study of bone graft used in fusion procedures and other procedures is necessary to define the magnitude of osteotoxic effects in the setting of fracture care or fusion procedures.
Figures
References
-
- American Association of Tissue Banks. AATB Standards for Tissue Banking. Available at: http://www.aatb.org/Standards-Updates-for-the-12th-Edition. Accessed October 26, 2015.
-
- Auederheide AC, Wittmers LE, Rapp G, Wallgren J. Anthropological applications of skeletal lead analysis. Am Anthropol. 1988;90:931–936. doi: 10.1525/aa.1988.90.4.02a00110. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical