Single Mutations in the VP2 300 Loop Region of the Three-Fold Spike of the Carnivore Parvovirus Capsid Can Determine Host Range
- PMID: 26512077
- PMCID: PMC4702700
- DOI: 10.1128/JVI.02636-15
Single Mutations in the VP2 300 Loop Region of the Three-Fold Spike of the Carnivore Parvovirus Capsid Can Determine Host Range
Abstract
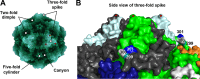
Sylvatic carnivores, such as raccoons, have recently been recognized as important hosts in the evolution of canine parvovirus (CPV), a pandemic pathogen of domestic dogs. Although viruses from raccoons do not efficiently bind the dog transferrin receptor (TfR) or infect dog cells, a single mutation changing an aspartic acid to a glycine at capsid (VP2) position 300 in the prototype raccoon CPV allows dog cell infection. Because VP2 position 300 exhibits extensive amino acid variation among the carnivore parvoviruses, we further investigated its role in determining host range by analyzing its diversity and evolution in nature and by creating a comprehensive set of VP2 position 300 mutants in infectious clones. Notably, some position 300 residues rendered CPV noninfectious for dog, but not cat or fox, cells. Changes of adjacent residues (residues 299 and 301) were also observed often after cell culture passage in different hosts, and some of the mutations mimicked changes seen in viruses recovered from natural infections of alternative hosts, suggesting that compensatory mutations were selected to accommodate the new residue at position 300. Analysis of the TfRs of carnivore hosts used in the experimental evolution studies demonstrated that their glycosylation patterns varied, including a glycan present only on the domestic dog TfR that dictates susceptibility to parvoviruses. Overall, there were significant differences in the abilities of viruses with alternative position 300 residues to bind TfRs and infect different carnivore hosts, demonstrating that the process of infection is highly host dependent and that VP2 position 300 is a key determinant of host range.
Importance: Although the emergence and pandemic spread of canine parvovirus (CPV) are well documented, the carnivore hosts and evolutionary pathways involved in its emergence remain enigmatic. We recently demonstrated that a region in the capsid structure of CPV, centered around VP2 position 300, varies after transfer to alternative carnivore hosts and may allow infection of previously nonsusceptible hosts in vitro. Here we show that VP2 position 300 is the most variable residue in the parvovirus capsid in nature, suggesting that it is a critical determinant in the cross-species transfer of viruses between different carnivores due to its interactions with the transferrin receptor to mediate infection. To this end, we demonstrated that there are substantial differences in receptor binding and infectivity of various VP2 position 300 mutants for different carnivore species and that single mutations in this region can influence whether a host is susceptible or refractory to virus infection.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures





References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

