Herpes Simplex Virus 1 Infection of Tree Shrews Differs from That of Mice in the Severity of Acute Infection and Viral Transcription in the Peripheral Nervous System
- PMID: 26512084
- PMCID: PMC4702684
- DOI: 10.1128/JVI.02258-15
Herpes Simplex Virus 1 Infection of Tree Shrews Differs from That of Mice in the Severity of Acute Infection and Viral Transcription in the Peripheral Nervous System
Abstract
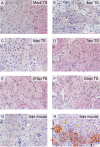
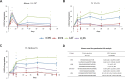
Studies of herpes simplex virus (HSV) infections of humans are limited by the use of rodent models such as mice, rabbits, and guinea pigs. Tree shrews (Tupaia belangeri chinensis) are small mammals indigenous to southwest Asia. At behavioral, anatomical, genomic, and evolutionary levels, tree shrews are much closer to primates than rodents are, and tree shrews are susceptible to HSV infection. Thus, we have studied herpes simplex virus 1 (HSV-1) infection in the tree shrew trigeminal ganglion (TG) following ocular inoculation. In situ hybridization, PCR, and quantitative reverse transcription-PCR (qRT-PCR) analyses confirm that HSV-1 latently infects neurons of the TG. When explant cocultivation of trigeminal ganglia was performed, the virus was recovered after 5 days of cocultivation with high efficiency. Swabbing the corneas of latently infected tree shrews revealed that tree shrews shed virus spontaneously at low frequencies. However, tree shrews differ significantly from mice in the expression of key HSV-1 genes, including ICP0, ICP4, and latency-associated transcript (LAT). In acutely infected tree shrew TGs, no level of ICP4 was observed, suggesting the absence of infection or a very weak, acute infection compared to that of the mouse. Immunofluorescence staining with ICP4 monoclonal antibody, and immunohistochemistry detection by HSV-1 polyclonal antibodies, showed a lack of viral proteins in tree shrew TGs during both acute and latent phases of infection. Cultivation of supernatant from homogenized, acutely infected TGs with RS1 cells also exhibited an absence of infectious HSV-1 from tree shrew TGs. We conclude that the tree shrew has an undetectable, or a much weaker, acute infection in the TGs. Interestingly, compared to mice, tree shrew TGs express high levels of ICP0 transcript in addition to LAT during latency. However, the ICP0 transcript remained nuclear, and no ICP0 protein could be seen during the course of mouse and tree shrew TG infections. Taken together, these observations suggest that the tree shrew TG infection differs significantly from the existing rodent models.
Importance: Herpes simplex viruses (HSVs) establish lifelong infection in more than 80% of the human population, and their reactivation leads to oral and genital herpes. Currently, rodent models are the preferred models for latency studies. Rodents are distant from primates and may not fully represent human latency. The tree shrew is a small mammal, a prosimian primate, indigenous to southwest Asia. In an attempt to further develop the tree shrew as a useful model to study herpesvirus infection, we studied the establishment of latency and reactivation of HSV-1 in tree shrews following ocular inoculation. We found that the latent virus, which resides in the sensory neurons of the trigeminal ganglion, could be stress reactivated to produce infectious virus, following explant cocultivation and that spontaneous reactivation could be detected by cell culture of tears. Interestingly, the tree shrew model is quite different from the mouse model of HSV infection, in that the virus exhibited only a mild acute infection following inoculation with no detectable infectious virus from the sensory neurons. The mild infection may be more similar to human infection in that the sensory neurons continue to function after herpes reactivation and the affected skin tissue does not lose sensation. Our findings suggest that the tree shrew is a viable model to study HSV latency.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures







References
-
- Bernstein DI, Stanberry LR, Harrison CJ, Kappes JC, Myers MG. 1986. Antibody response, recurrence patterns and subsequent herpes simplex virus type 2 (HSV-2) reinfection following initial HSV-2 infection of guinea pigs: effects of acyclovir. J Gen Virol 67:1601–1612. doi:10.1099/0022-1317-67-8-1601. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

