Changes in Regenerative Capacity through Lifespan
- PMID: 26512653
- PMCID: PMC4632807
- DOI: 10.3390/ijms161025392
Changes in Regenerative Capacity through Lifespan
Abstract
Most organisms experience changes in regenerative abilities through their lifespan. During aging, numerous tissues exhibit a progressive decline in homeostasis and regeneration that results in tissue degeneration, malfunction and pathology. The mechanisms responsible for this decay are both cell intrinsic, such as cellular senescence, as well as cell-extrinsic, such as changes in the regenerative environment. Understanding how these mechanisms impact on regenerative processes is essential to devise therapeutic approaches to improve tissue regeneration and extend healthspan. This review offers an overview of how regenerative abilities change through lifespan in various organisms, the factors that underlie such changes and the avenues for therapeutic intervention. It focuses on established models of mammalian regeneration as well as on models in which regenerative abilities do not decline with age, as these can deliver valuable insights for our understanding of the interplay between regeneration and aging.
Keywords: aging; axolotl; newt; planaria; regeneration; reprogramming; senescence; stem cells; zebrafish.
Figures

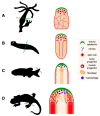
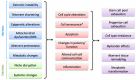

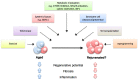
References
-
- Finch C.E. Longevity, Senescence and the Genome. The University of Chicago Press; Chicago, IL, USA: 1994.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

