Fucosylation and protein glycosylation create functional receptors for cholera toxin
- PMID: 26512888
- PMCID: PMC4686427
- DOI: 10.7554/eLife.09545
Fucosylation and protein glycosylation create functional receptors for cholera toxin
Abstract
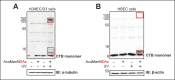
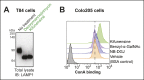
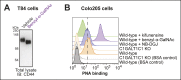
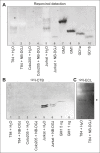
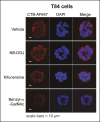
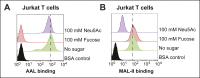
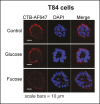
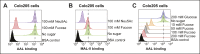
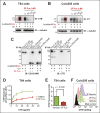
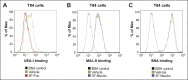
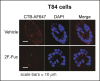
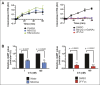
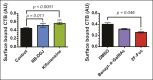
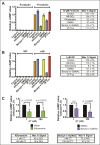
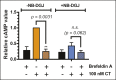
Cholera toxin (CT) enters and intoxicates host cells after binding cell surface receptors using its B subunit (CTB). The ganglioside (glycolipid) GM1 is thought to be the sole CT receptor; however, the mechanism by which CTB binding to GM1 mediates internalization of CT remains enigmatic. Here we report that CTB binds cell surface glycoproteins. Relative contributions of gangliosides and glycoproteins to CTB binding depend on cell type, and CTB binds primarily to glycoproteins in colonic epithelial cell lines. Using a metabolically incorporated photocrosslinking sugar, we identified one CTB-binding glycoprotein and demonstrated that the glycan portion of the molecule, not the protein, provides the CTB interaction motif. We further show that fucosylated structures promote CTB entry into a colonic epithelial cell line and subsequent host cell intoxication. CTB-binding fucosylated glycoproteins are present in normal human intestinal epithelia and could play a role in cholera.
Keywords: biochemistry; cholera; endocytosis; epithelial cells; glycolipids/gangliosides; glycoprotein; infectious disease; microbiology; none; toxins.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures








References
-
- Aman AT, Fraser S, Merritt EA, Rodigherio C, Kenny M, Ahn M, Hol WGJ, Williams NA, Lencer WI, Hirst TR. A mutant cholera toxin b subunit that binds GM1- ganglioside but lacks immunomodulatory or toxic activity. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:8536–8541. doi: 10.1073/pnas.161273098. - DOI - PMC - PubMed
-
- Angstrom J, Teneberg S, Karlsson KA. Delineation and comparison of ganglioside-binding Epitopes for the toxins of vibrio cholerae, Escherichia coli, and clostridium tetani: evidence for overlapping epitopes. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:11859–11863. doi: 10.1073/pnas.91.25.11859. - DOI - PMC - PubMed
-
- Balanzino LE, Barra JL, Monferran CG, Cumar FA. Differential interaction of escherichia coli heat-labile toxin and cholera toxin with pig intestinal brush border glycoproteins depending on their ABH and related blood group antigenic determinants. Infection and Immunity. 1994;62:1460–1464. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

