Peptide/protein vaccine delivery system based on PLGA particles
- PMID: 26513024
- PMCID: PMC4964737
- DOI: 10.1080/21645515.2015.1102804
Peptide/protein vaccine delivery system based on PLGA particles
Abstract
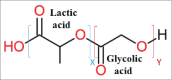
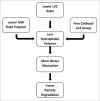
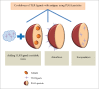
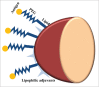
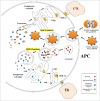
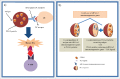
Due to the excellent safety profile of poly (D,L-lactide-co-glycolide) (PLGA) particles in human, and their biodegradability, many studies have focused on the application of PLGA particles as a controlled-release vaccine delivery system. Antigenic proteins/peptides can be encapsulated into or adsorbed to the surface of PLGA particles. The gradual release of loaded antigens from PLGA particles is necessary for the induction of efficient immunity. Various factors can influence protein release rates from PLGA particles, which can be defined intrinsic features of the polymer, particle characteristics as well as protein and environmental related factors. The use of PLGA particles encapsulating antigens of different diseases such as hepatitis B, tuberculosis, chlamydia, malaria, leishmania, toxoplasma and allergy antigens will be described herein. The co-delivery of antigens and immunostimulants (IS) with PLGA particles can prevent the systemic adverse effects of immunopotentiators and activate both dendritic cells (DCs) and natural killer (NKs) cells, consequently enhancing the therapeutic efficacy of antigen-loaded PLGA particles. We will review co-delivery of different TLR ligands with antigens in various models, highlighting the specific strengths and weaknesses of the system. Strategies to enhance the immunotherapeutic effect of DC-based vaccine using PLGA particles can be designed to target DCs by functionalized PLGA particle encapsulating siRNAs of suppressive gene, and disease specific antigens. Finally, specific examples of cellular targeting where decorating the surface of PLGA particles target orally administrated vaccine to M-cells will be highlighted.
Keywords: PLGA particle; TLR ligands; delivery system; micro/nano particles; protein/peptide vaccine.
Figures



References
-
- Jain S, O'Hagan DT, Singh M. The long-term potential of biodegradable poly(lactide-co-glycolide) microparticles as the next-generation vaccine adjuvant. Expert Rev Vaccines 2011; 10:1731-42; PMID:22085176; http://dx.doi.org/10.1586/erv.11.126 - DOI - PubMed
-
- Makadia HK, Siegel SJ. Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers 2011; 3:1377-97; PMID:22577513; http://dx.doi.org/10.3390/polym3031377 - DOI - PMC - PubMed
-
- Semete B, Booysen L, Lemmer Y, Kalombo L, Katata L, Verschoor J, Swai HS. In vivo evaluation of the biodistribution and safety of PLGA nanoparticles as drug delivery systems. Nanomedicine 2010; 6:662-71; http://dx.doi.org/10.1016/j.nano.2010.02.002 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
