Distinct Macrophage Fates after in vitro Infection with Different Species of Leishmania: Induction of Apoptosis by Leishmania (Leishmania) amazonensis, but Not by Leishmania (Viannia) guyanensis
- PMID: 26513474
- PMCID: PMC4626090
- DOI: 10.1371/journal.pone.0141196
Distinct Macrophage Fates after in vitro Infection with Different Species of Leishmania: Induction of Apoptosis by Leishmania (Leishmania) amazonensis, but Not by Leishmania (Viannia) guyanensis
Abstract
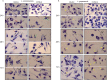
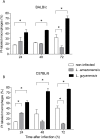
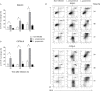
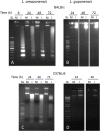
Leishmania is an intracellular parasite in vertebrate hosts, including man. During infection, amastigotes replicate inside macrophages and are transmitted to healthy cells, leading to amplification of the infection. Although transfer of amastigotes from infected to healthy cells is a crucial step that may shape the outcome of the infection, it is not fully understood. Here we compare L. amazonensis and L. guyanensis infection in C57BL/6 and BALB/c mice and investigate the fate of macrophages when infected with these species of Leishmania in vitro. As previously shown, infection of mice results in distinct outcomes: L. amazonensis causes a chronic infection in both strains of mice (although milder in C57BL/6), whereas L. guyanensis does not cause them disease. In vitro, infection is persistent in L. amazonensis-infected macrophages whereas L. guyanensis growth is controlled by host cells from both strains of mice. We demonstrate that, in vitro, L. amazonensis induces apoptosis of both C57BL/6 and BALB/c macrophages, characterized by PS exposure, DNA cleavage into nucleosomal size fragments, and consequent hypodiploidy. None of these signs were seen in macrophages infected with L. guyanensis, which seem to die through necrosis, as indicated by increased PI-, but not Annexin V-, positive cells. L. amazonensis-induced macrophage apoptosis was associated to activation of caspases-3, -8 and -9 in both strains of mice. Considering these two species of Leishmania and strains of mice, macrophage apoptosis, induced at the initial moments of infection, correlates with chronic infection, regardless of its severity. We present evidence suggestive that macrophages phagocytize L. amazonensis-infected cells, which has not been verified so far. The ingestion of apoptotic infected macrophages by healthy macrophages could be a way of amastigote spreading, leading to the establishment of infection.
Conflict of interest statement
Figures



References
-
- World Health Organization. Leishmaniasis: the disease and its epidemiology. 2014. Available: http://www.who.int/leishmaniasis/disease_epidemiology/en. Accessed January 2015.
-
- Kedzierski L, Evans KJ. Immune response during cutaneous and visceral leishmaniasis. 2014. Parasitology 30: 1–19. - PubMed
-
- World Health Organization. Global Alert and Response. 2014. Available: http://www.who.int/csr/resources/publications/CSR_ISR_2000_1leish/en/. Accessed January 2015.
-
- Gontijo B, de Carvalho ML. Leishmaniose Tegumentar Americana. Rev Soc Bras Med Trop 2003; 36:71–80. - PubMed
-
- Abreu-Silva AL, Calabrese KS, Cupolilo SMN, Cardoso FO, Souza CSF, Gonçalves da Costa SC. Histopathological studies of visceralized Leishmania (Leishmania) amazonensis in mice experimentally infected. Vet Parasitol 2004; 121: 179–187. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

