In Vivo Visualization of Endoplasmic Reticulum Stress in the Retina Using the ERAI Reporter Mouse
- PMID: 26513501
- PMCID: PMC4627472
- DOI: 10.1167/iovs.15-16969
In Vivo Visualization of Endoplasmic Reticulum Stress in the Retina Using the ERAI Reporter Mouse
Abstract
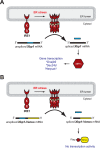
Purpose: Endoplasmic reticulum (ER) stress activates inositol requiring enzyme 1 (IRE1), a key regulator of the unfolded protein response. The ER stress activated indicator (ERAI) transgenic mouse expresses a yellow fluorescent GFP variant (Venus) when IRE1 is activated by ER stress. We tested whether ERAI mice would allow for real-time longitudinal studies of ER stress in living mouse eyes.
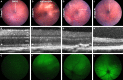
Methods: We chemically and genetically induced ER stress, and qualitatively and quantitatively studied the Venus signal by fluorescence ophthalmoscopy. We determined retinal cell types that contribute to the signal by immunohistology, and we performed molecular and biochemical assays using whole retinal lysates to assess activity of the IRE1 pathway.
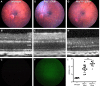
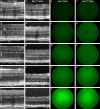
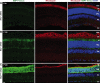
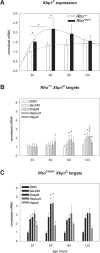
Results: We found qualitative increase in vivo in fluorescence signal at sites of intravitreal tunicamycin injection in ERAI eyes, and quantitative increase in ERAI mice mated to RhoP23H mice expressing ER stress-inducing misfolded rhodopsin protein. As expected, we found that increased Venus signal arose primarily from photoreceptors in RhoP23H/+;ERAI mice. We found increased Xbp1S and XBP1s transcriptional target mRNA levels in RhoP23H/+;ERAI retinas compared to Rho+/+;ERAI retinas, and that Venus signal increased in ERAI retinas as a function of age.
Conclusions: Fluorescence ophthalmoscopy of ERAI mice enables in vivo visualization of retinas undergoing ER stress. ER stress activated indicator mice enable identification of individual retinal cells undergoing ER stress by immunohistochemistry. ER stress activated indicator mice show higher Venus signal at older ages, likely arising from amplification of basal retinal ER stress levels by GFP's inherent stability.
Figures

References
-
- Alberts B. Molecular Biology of the Cell. 5th ed. New York, NY: Garland Science; 2008: 1 v. (various pages).
-
- Walter P,, Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science. 2011. ; 334: 1081–1086. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- EY020846/EY/NEI NIH HHS/United States
- P30 NS047101/NS/NINDS NIH HHS/United States
- P30 EY022589/EY/NEI NIH HHS/United States
- I01 BX002284/BX/BLRD VA/United States
- R01 EY020846/EY/NEI NIH HHS/United States
- F32 EY006842/EY/NEI NIH HHS/United States
- P30EY022589/EY/NEI NIH HHS/United States
- R01 EY006842/EY/NEI NIH HHS/United States
- R01 EY019514/EY/NEI NIH HHS/United States
- NS088485/NS/NINDS NIH HHS/United States
- R01 EY001919/EY/NEI NIH HHS/United States
- EY001919/EY/NEI NIH HHS/United States
- P30EY002162/EY/NEI NIH HHS/United States
- R01 NS088485/NS/NINDS NIH HHS/United States
- EY006842/EY/NEI NIH HHS/United States
- EY019514/EY/NEI NIH HHS/United States
- P30 EY002162/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

