Selective Activation of Nociceptor TRPV1 Channel and Reversal of Inflammatory Pain in Mice by a Novel Coumarin Derivative Muralatin L from Murraya alata
- PMID: 26515068
- PMCID: PMC4705384
- DOI: 10.1074/jbc.M115.654392
Selective Activation of Nociceptor TRPV1 Channel and Reversal of Inflammatory Pain in Mice by a Novel Coumarin Derivative Muralatin L from Murraya alata
Abstract
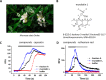
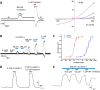
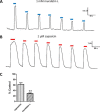
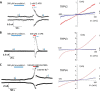
Coumarin and its derivatives are fragrant natural compounds isolated from the genus Murraya that are flowering plants widely distributed in East Asia, Australia, and the Pacific Islands. Murraya plants have been widely used as medicinal herbs for relief of pain, such as headache, rheumatic pain, toothache, and snake bites. However, little is known about their analgesic components and the molecular mechanism underlying pain relief. Here, we report the bioassay-guided fractionation and identification of a novel coumarin derivative, named muralatin L, that can specifically activate the nociceptor transient receptor potential vanilloid 1 (TRPV1) channel and reverse the inflammatory pain in mice through channel desensitization. Muralatin L was identified from the active extract of Murraya alata against TRPV1 transiently expressed in HEK-293T cells in fluorescent calcium FlexStation assay. Activation of TRPV1 current by muralatin L and its selectivity were further confirmed by whole-cell patch clamp recordings of TRPV1-expressing HEK-293T cells and dorsal root ganglion neurons isolated from mice. Furthermore, muralatin L could reverse inflammatory pain induced by formalin and acetic acid in mice but not in TRPV1 knock-out mice. Taken together, our findings show that muralatin L specifically activates TRPV1 and reverses inflammatory pain, thus highlighting the potential of coumarin derivatives from Murraya plants for pharmaceutical and medicinal applications such as pain therapy.
Keywords: calcium; calcium imaging; drug discovery; drug screening; ion channel; neuron; pain; transient receptor potential channels (TRP channels).
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures




References
-
- Song L. R. (1999) Chinese Materia Medica (Zhong Hua Ben Cao) Vol. 4, pp. 961–948, Shanghai Science & Technology Press, Shanghai, China
-
- Nahin R. L., Barnes P. M., Stussman B. J., and Bloom B. (2009) Costs of complementary and alternative medicine (CAM) and frequency of visits to CAM practitioners: United States 2007. Natl. Health Stat. Report 30, 1–14 - PubMed
-
- Caterina M. J., Schumacher M. A., Tominaga M., Rosen T. A., Levine J. D., and Julius D. (1997) The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389, 816–824 - PubMed
-
- Yang B. H., Piao Z. G., Kim Y. B., Lee C. H., Lee J. K., Park K., Kim J. S., and Oh S. B. (2003) Activation of vanilloid receptor 1 (VR1) by eugenol. J. Dent. Res. 82, 781–785 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

