Mechanisms of Calmodulin Regulation of Different Isoforms of Kv7.4 K+ Channels
- PMID: 26515070
- PMCID: PMC4732230
- DOI: 10.1074/jbc.M115.668236
Mechanisms of Calmodulin Regulation of Different Isoforms of Kv7.4 K+ Channels
Abstract
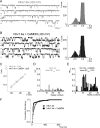
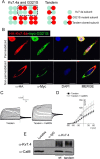
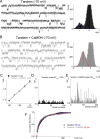
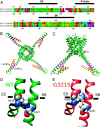
Calmodulin (CaM), a Ca(2+)-sensing protein, is constitutively bound to IQ domains of the C termini of human Kv7 (hKv7, KCNQ) channels to mediate Ca(2+)-dependent reduction of Kv7 currents. However, the mechanism remains unclear. We report that CaM binds to two isoforms of the hKv7.4 channel in a Ca(2+)-independent manner but that only the long isoform (hKv7.4a) is regulated by Ca(2+)/CaM. Ca(2+)/CaM mediate reduction of the hKv7.4a channel by decreasing the channel open probability and altering activation kinetics. We took advantage of a known missense mutation (G321S) that has been linked to progressive hearing loss to further examine the inhibitory effects of Ca(2+)/CaM on the Kv7.4 channel. Using multidisciplinary techniques, we demonstrate that the G321S mutation may destabilize CaM binding, leading to a decrease in the inhibitory effects of Ca(2+) on the channels. Our study utilizes an expression system to dissect the biophysical properties of the WT and mutant Kv7.4 channels. This report provides mechanistic insights into the critical roles of Ca(2+)/CaM regulation of the Kv7.4 channel under physiological and pathological conditions.
Keywords: calcium; calmodulin (CaM); hearing; ion channel; potassium channel.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures



References
-
- Yus-Najera E., Santana-Castro I., and Villarroel A. (2002) The identification and characterization of a noncontinuous calmodulin-binding site in noninactivating voltage-dependent KCNQ potassium channels. J. Biol. Chem. 277, 28545–28553 - PubMed
-
- Mooseker M. S., and Cheney R. E. (1995) Unconventional myosins. Annu. Rev. Cell Dev. Biol. 11, 633–675 - PubMed
-
- Wingo T. L., Shah V. N., Anderson M. E., Lybrand T. P., Chazin W. J., and Balser J. R. (2004) An EF-hand in the sodium channel couples intracellular calcium to cardiac excitability. Nat. Struct. Mol. Biol. 11, 219–225 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

