Allelic variation contributes to bacterial host specificity
- PMID: 26515720
- PMCID: PMC4640099
- DOI: 10.1038/ncomms9754
Allelic variation contributes to bacterial host specificity
Erratum in
-
Corrigendum: Allelic variation contributes to bacterial host specificity.Nat Commun. 2017 Aug 8;8:15229. doi: 10.1038/ncomms15229. Nat Commun. 2017. PMID: 28786410 Free PMC article.
Abstract
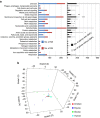
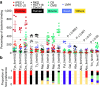
Understanding the molecular parameters that regulate cross-species transmission and host adaptation of potential pathogens is crucial to control emerging infectious disease. Although microbial pathotype diversity is conventionally associated with gene gain or loss, the role of pathoadaptive nonsynonymous single-nucleotide polymorphisms (nsSNPs) has not been systematically evaluated. Here, our genome-wide analysis of core genes within Salmonella enterica serovar Typhimurium genomes reveals a high degree of allelic variation in surface-exposed molecules, including adhesins that promote host colonization. Subsequent multinomial logistic regression, MultiPhen and Random Forest analyses of known/suspected adhesins from 580 independent Typhimurium isolates identifies distinct host-specific nsSNP signatures. Moreover, population and functional analyses of host-associated nsSNPs for FimH, the type 1 fimbrial adhesin, highlights the role of key allelic residues in host-specific adherence in vitro. Together, our data provide the first concrete evidence that functional differences between allelic variants of bacterial proteins likely contribute to pathoadaption to diverse hosts.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


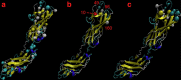

References
-
- Shi Y., Wu Y., Zhang W., Qi J. & Gao G. F. Enabling the ‘host jump’: structural determinants of receptor-binding specificity in influenza A viruses. Nat. Rev. Microbiol. 12, 822–831 (2014). - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

