Divergent C-H Insertion-Cyclization Cascades of N-Allyl Ynamides
- PMID: 26515958
- PMCID: PMC4832826
- DOI: 10.1002/anie.201507167
Divergent C-H Insertion-Cyclization Cascades of N-Allyl Ynamides
Abstract
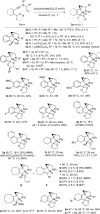
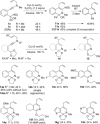
Gold carbene reactivity patterns were accessed by ynamide insertion into a C(sp(3) )H bond. A substantial increase in molecular complexity occurred through the cascade polycyclization of N-allyl ynamides to form fused nitrogen-heterocycle scaffolds. Exquisite selectivity was observed despite several competing pathways in an efficient gold-catalyzed synthesis of densely functionalized C(sp(3) )-rich polycycles and a copper-catalyzed synthesis of fused pyridine derivatives. The respective gold-keteniminium and ketenimine activation pathways have been explored through a structure-reactivity study, and isotopic labeling identified turnover-limiting CH bond-cleavage in both processes.
Keywords: carbenes; cyclopropanation; gold; nitrogen heterocycles; ynamides.
© 2015 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA. This is an open access article under the terms of the Creative Commons Attribution License, which permits use, distribution and reproduction in any medium, provided the original work is properly cited.
Figures



References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

