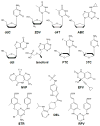
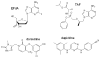Resistance to reverse transcriptase inhibitors used in the treatment and prevention of HIV-1 infection
- PMID: 26517190
- PMCID: PMC4813512
- DOI: 10.2217/fmb.15.106
Resistance to reverse transcriptase inhibitors used in the treatment and prevention of HIV-1 infection
Abstract
Inhibitors that target the retroviral enzyme reverse transcriptase (RT) have played an indispensable role in the treatment and prevention of HIV-1 infection. They can be grouped into two distinct therapeutic groups, namely the nucleoside and nucleotide RT inhibitors (NRTIs), and the non-nucleoside RT inhibitors (NNRTIs). NRTIs form the backbones of most first- and second-line antiretroviral therapy (ART) regimens formulated for the treatment of HIV-1 infection. They are also used to prevent mother-to-child transmission, and as pre-exposure prophylaxis in individuals at risk of HIV-1 infection. The NNRTIs nevirapine (NVP), efavirenz and rilpivirine also used to form part of first-line ART regimens, although this is no longer recommended, while etravirine can be used in salvage ART regimens. A single-dose of NVP administered to both mother and child has routinely been used in resource-limited settings to reduce the rate of HIV-1 transmission. Unfortunately, the development of HIV-1 resistance to RT inhibitors can compromise the efficacy of these antiviral drugs in both the treatment and prevention arenas. Here, we provide an up-to-date review on drug-resistance mutations in HIV-1 RT, and discuss their cross-resistance profiles, molecular mechanisms and clinical significance.
Keywords: HIV; mutations; non-nucleoside; nucleoside; resistance; reverse transcriptase.
Figures
References
-
- Chen R, Quinones-Mateu ME, Mansky LM. Drug resistance, virus fitness and HIV-1 mutagenesis. Curr Pharm Des. 2004;10:4065–4070. - PubMed
-
- Meyer PR, Matsuura SE, Mian AM, et al. A mechanism of AZT resistance: an increase in nucleotide-dependent primer unblocking by mutant HIV-1 reverse transcriptase. Mol Cell. 1998;4:35–43. - PubMed
-
- Feng JY, Anderson KS. Mechanistic studies examining the efficiency and fidelity of DNA synthesis by the 3TC-resistant mutant (184V) of HIV-1 reverse transcriptase. Biochemistry. 1998;38:9440–9448. - PubMed
-
- Selmi B, Boretto J, Sarfati SR, et al. Mechanism-based suppression of dideoxynucleotide resistance by K65R human immunodeficiency virus reverse transcriptase using an alpha-boranophosphate nucleoside analogue. J Biol Chem. 2001;276:48466–48472. - PubMed
-
- Deval J, Navarro JM, Selmi B, et al. A loss of viral replicative capacity correlates with altered DNA polymerization kinetics by the human immunodeficiency virus reverse transcriptase bearing the K65R and L74V dideoxynucleoside resistance substitutions. J Biol Chem. 2004;279:25489–25496. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources