A randomised double-blind clinical trial of two yellow fever vaccines prepared with substrains 17DD and 17D-213/77 in children nine-23 months old
- PMID: 26517656
- PMCID: PMC4667580
- DOI: 10.1590/0074-02760150176
A randomised double-blind clinical trial of two yellow fever vaccines prepared with substrains 17DD and 17D-213/77 in children nine-23 months old
Abstract
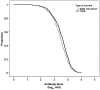
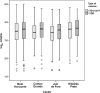
This randomised, double-blind, multicentre study with children nine-23 months old evaluated the immunogenicity of yellow fever (YF) vaccines prepared with substrains 17DD and 17D-213/77. YF antibodies were titered before and 30 or more days after vaccination. Seropositivity and seroconversion were analysed according to the maternal serological status and the collaborating centre. A total of 1,966 children were randomised in the municipalities of the states of Mato Grosso do Sul, Minas Gerais and São Paulo and blood samples were collected from 1,714 mothers. Seropositivity was observed in 78.6% of mothers and 8.9% of children before vaccination. After vaccination, seropositivity rates of 81.9% and 83.2%, seroconversion rates of 84.8% and 85.8% and rates of a four-fold increase over the pre-vaccination titre of 77.6% and 81.8% were observed in the 17D-213/77 and 17DD subgroups, respectively. There was no association with maternal immunity. Among children aged 12 months or older, the seroconversion rates of 69% were associated with concomitant vaccination against measles, mumps and rubella. The data were not conclusive regarding the interference of maternal immunity in the immune response to the YF vaccine, but they suggest interference from other vaccines. The failures in seroconversion after vaccination support the recommendation of a booster dose in children within 10 years of the first dose.
Figures
References
-
- Adu FD, Omotade OO, Oyedele OI, Ikusika O, Odemuyiwa SO, Onoja AL. Field trial of combined yellow fever and measles vaccines among children in Nigeria. East Afr Med J. 1996;73:579–582. - PubMed
-
- Belmusto-Worn VE, Sanchez JL, McCarthy K, Nichols R, Bautista CT, Magill AJ, Pastor-Cauna G, Echevarria C, Laguna-Torres VA, Samame BK, Baldeon MA, Burans JP, Olson JG, Bedford P, Kitchener S, Monath TP. Randomized, double-blind, Phase III, pivotal field trial of the comparative immunogenicity, safety and tolerability of two yellow fever 17D vaccines (Arilvax and YF-VAX) in healthy infants and children in Peru. Am J Trop Med Hyg. 2005;72:189–197. - PubMed
-
- Camacho LAB, Freire MS, Leal MLF, Aguiar SG, Nascimento JP, Igushi T, Lozana JA, Farias RHG. Collaborative Group for the Study of Yellow Fever Vaccines. Immunogenicity of WHO-17D and Brazilian 17DD yellow fever vaccines: a randomized trial. Rev Saude Publica. 2004;38:671–678. - PubMed
-
- Collaborative Group for Studies with Yellow Fever Randomized, double-blind, multicenter study of the immunogenicity and reactogenicity of 17DD and WHO 17D-213/77 yellow fever vaccines in children: implications for the Brazilian National Immunization Program. Vaccine. 2007;25:3118–3123. - PubMed
-
- Fleiss JL. The design and analysis of clinical experiments. John Wiley & Sons; New York: 1986. 432 pp
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

