In Vivo Quantification of Peroxisome Tethering to Chloroplasts in Tobacco Epidermal Cells Using Optical Tweezers
- PMID: 26518344
- PMCID: PMC4704594
- DOI: 10.1104/pp.15.01529
In Vivo Quantification of Peroxisome Tethering to Chloroplasts in Tobacco Epidermal Cells Using Optical Tweezers
Abstract
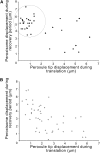
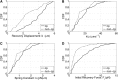
Peroxisomes are highly motile organelles that display a range of motions within a short time frame. In static snapshots, they can be juxtaposed to chloroplasts, which has led to the hypothesis that they are physically interacting. Here, using optical tweezers, we tested the dynamic physical interaction in vivo. Using near-infrared optical tweezers combined with TIRF microscopy, we were able to trap peroxisomes and approximate the forces involved in chloroplast association in vivo in tobacco (Nicotiana tabacum) and observed weaker tethering to additional unknown structures within the cell. We show that chloroplasts and peroxisomes are physically tethered through peroxules, a poorly described structure in plant cells. We suggest that peroxules have a novel role in maintaining peroxisome-organelle interactions in the dynamic environment. This could be important for fatty acid mobilization and photorespiration through the interaction with oil bodies and chloroplasts, highlighting a fundamentally important role for organelle interactions for essential biochemistry and physiological processes.
© 2016 American Society of Plant Biologists. All Rights Reserved.
Figures


References
-
- Andersson MX, Goksör M, Sandelius AS (2007) Optical manipulation reveals strong attracting forces at membrane contact sites between endoplasmic reticulum and chloroplasts. J Biol Chem 282: 1170–1174 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

