Telomerase Deficiency Causes Alveolar Stem Cell Senescence-associated Low-grade Inflammation in Lungs
- PMID: 26518879
- PMCID: PMC4692211
- DOI: 10.1074/jbc.M115.681619
Telomerase Deficiency Causes Alveolar Stem Cell Senescence-associated Low-grade Inflammation in Lungs
Abstract
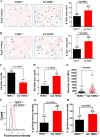
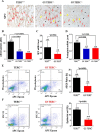
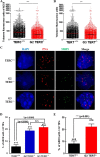
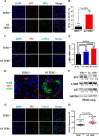
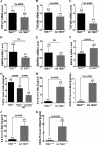
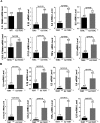
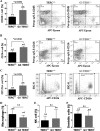
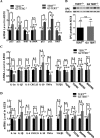
Mutations of human telomerase RNA component (TERC) and telomerase reverse transcriptase (TERT) are associated with a subset of lung aging diseases, but the mechanisms by which TERC and TERT participate in lung diseases remain unclear. In this report, we show that knock-out (KO) of the mouse gene Terc or Tert causes pulmonary alveolar stem cell replicative senescence, epithelial impairment, formation of alveolar sacs, and characteristic inflammatory phenotype. Deficiency in TERC or TERT causes a remarkable elevation in various proinflammatory cytokines, including IL-1, IL-6, CXCL15 (human IL-8 homolog), IL-10, TNF-α, and monocyte chemotactic protein 1 (chemokine ligand 2 (CCL2)); decrease in TGF-β1 and TGFβRI receptor in the lungs; and spillover of IL-6 and CXCL15 into the bronchoalveolar lavage fluids. In addition to increased gene expressions of α-smooth muscle actin and collagen 1α1, suggesting myofibroblast differentiation, TERC deficiency also leads to marked cellular infiltrations of a mononuclear cell population positive for the leukocyte common antigen CD45, low-affinity Fc receptor CD16/CD32, and pattern recognition receptor CD11b in the lungs. Our data demonstrate for the first time that telomerase deficiency triggers alveolar stem cell replicative senescence-associated low-grade inflammation, thereby driving pulmonary premature aging, alveolar sac formation, and fibrotic lesion.
Keywords: alveolar senescence; chronic obstructive pulmonary disease (COPD); lung aging; proinflammatory cytokines; pulmonary fibrosis; telomerase; telomerase reverse transcriptase (TERT); telomere; telomeres.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures

References
-
- Blackburn E. H. (2005) Telomeres and telomerase: their mechanisms of action and the effects of altering their functions. FEBS Lett. 579, 859–862 - PubMed
-
- Kong C. M., Lee X. W., and Wang X. (2013) Telomere shortening in human diseases. FEBS J. 280, 3180–3193 - PubMed
-
- Mitchell J. R., Wood E., and Collins K. (1999) A telomerase component is defective in the human disease dyskeratosis congenita. Nature 402, 551–555 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

