Dexamethasone intravitreal implant in previously treated patients with diabetic macular edema: subgroup analysis of the MEAD study
- PMID: 26519345
- PMCID: PMC4628378
- DOI: 10.1186/s12886-015-0148-2
Dexamethasone intravitreal implant in previously treated patients with diabetic macular edema: subgroup analysis of the MEAD study
Abstract
Background: Dexamethasone intravitreal implant 0.7 mg (DEX 0.7) was approved for treatment of diabetic macular edema (DME) after demonstration of its efficacy and safety in the MEAD registration trials. We performed subgroup analysis of MEAD study results to evaluate the efficacy and safety of DEX 0.7 treatment in patients with previously treated DME.
Methods: Three-year, randomized, sham-controlled phase 3 study in patients with DME, best-corrected visual acuity (BCVA) of 34-68 Early Treatment Diabetic Retinopathy Study letters (20/200-20/50 Snellen equivalent), and central retinal thickness (CRT) ≥ 300 μm measured by time-domain optical coherence tomography. Patients were randomized to 1 of 2 doses of DEX (0.7 mg or 0.35 mg), or to sham procedure, with retreatment no more than every 6 months. The primary endpoint was ≥ 15-letter gain in BCVA at study end. Average change in BCVA and CRT from baseline during the study (area-under-the-curve approach) and adverse events were also evaluated. The present subgroup analysis evaluated outcomes in patients randomized to DEX 0.7 (marketed dose) or sham based on prior treatment for DME at study entry.
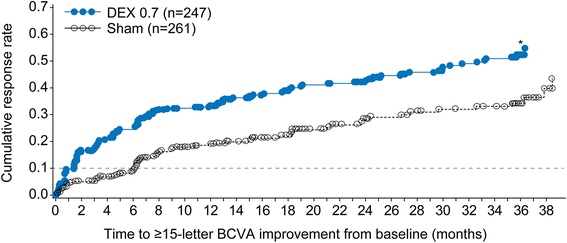
Results: Baseline characteristics of previously treated DEX 0.7 (n = 247) and sham (n = 261) patients were similar. In the previously treated subgroup, mean number of treatments over 3 years was 4.1 for DEX 0.7 and 3.2 for sham, 21.5% of DEX 0.7 patients versus 11.1 % of sham had ≥ 15-letter BCVA gain from baseline at study end (P = 0.002), mean average BCVA change from baseline was +3.2 letters with DEX 0.7 versus +1.5 letters with sham (P = 0.024), and mean average CRT change from baseline was -126.1 μm with DEX 0.7 versus -39.0 μm with sham (P < .001). Cataract-related adverse events were reported in 70.3% of baseline phakic patients in the previously treated DEX 0.7 subgroup; vision gains were restored following cataract surgery.
Conclusions: DEX 0.7 significantly improved visual and anatomic outcomes in patients with DME previously treated with laser, intravitreal anti-vascular endothelial growth factor, intravitreal triamcinolone acetonide, or a combination of these therapies. The safety profile of DEX 0.7 in previously treated patients was similar to its safety profile in the total study population.
Trial registration: ClinicalTrials.gov NCT00168337 and NCT00168389, registered 12 September 2005.
Figures

References
-
- Miyamoto K, Khosrof S, Bursell SE, Rohan R, Murata T, Clermont AC, et al. Prevention of leukostasis and vascular leakage in streptozotocin-induced diabetic retinopathy via intercellular adhesion molecule-1 inhibition. Proc Natl Acad Sci U S A. 1999;96:10836–41. doi: 10.1073/pnas.96.19.10836. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

