Zebrafish lacking functional DNA polymerase gamma survive to juvenile stage, despite rapid and sustained mitochondrial DNA depletion, altered energetics and growth
- PMID: 26519465
- PMCID: PMC4666367
- DOI: 10.1093/nar/gkv1139
Zebrafish lacking functional DNA polymerase gamma survive to juvenile stage, despite rapid and sustained mitochondrial DNA depletion, altered energetics and growth
Abstract
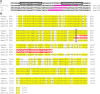
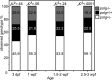
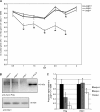
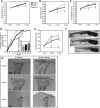
DNA polymerase gamma (POLG) is essential for replication and repair of mitochondrial DNA (mtDNA). Mutations in POLG cause mtDNA instability and a diverse range of poorly understood human diseases. Here, we created a unique Polg animal model, by modifying polg within the critical and highly conserved polymerase domain in zebrafish. polg(+/-) offspring were indistinguishable from WT siblings in multiple phenotypic and biochemical measures. However, polg(-/-) mutants developed severe mtDNA depletion by one week post-fertilization (wpf), developed slowly and had regenerative defects, yet surprisingly survived up to 4 wpf. An in vivo mtDNA polymerase activity assay utilizing ethidium bromide (EtBr) to deplete mtDNA, showed that polg(+/-) and WT zebrafish fully recover mtDNA content two weeks post-EtBr removal. EtBr further reduced already low levels of mtDNA in polg(-/-) animals, but mtDNA content did not recover following release from EtBr. Despite significantly decreased respiration that corresponded with tissue-specific levels of mtDNA, polg(-/-) animals had WT levels of ATP and no increase in lactate. This zebrafish model of mitochondrial disease now provides unique opportunities for studying mtDNA instability from multiple angles, as polg(-/-) mutants can survive to juvenile stage, rather than lose viability in embryogenesis as seen in Polg mutant mice.
© The Author(s) 2015. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures


References
-
- Zheng W., Khrapko K., Coller H.A., Thilly W.G., Copeland W.C. Origins of human mitochondrial point mutations as DNA polymerase gamma-mediated errors. Mutat. Res. 2006;599:11–20. - PubMed
-
- Cohen B.H., Chinnery P.F., Copeland W.C. In: GeneReviews(R) Pagon R.A., Adam M.P., Ardinger H.H., Wallace S.E., Amemiya A., Bean L.J.H., Bird T.D., Dolan C.R., Fong C.T., Smith R.J.H., editors. Seattle, Seattle (WA): Univeristy of Washington; 2010. (eds.)
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

