β-Glucan-Activated Human B Lymphocytes Participate in Innate Immune Responses by Releasing Proinflammatory Cytokines and Stimulating Neutrophil Chemotaxis
- PMID: 26519534
- PMCID: PMC4655155
- DOI: 10.4049/jimmunol.1500559
β-Glucan-Activated Human B Lymphocytes Participate in Innate Immune Responses by Releasing Proinflammatory Cytokines and Stimulating Neutrophil Chemotaxis
Abstract
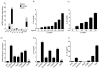
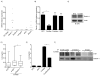
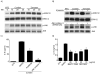
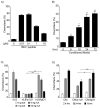
B lymphocytes play an essential regulatory role in the adaptive immune response through Ab production during infection. A less known function of B lymphocytes is their ability to respond directly to infectious Ags through stimulation of pattern recognition receptors expressed on their surfaces. β-Glucans are carbohydrates present in the cell wall of many pathogenic fungi that can be detected in the peripheral blood of patients during infection. They have been shown to participate in the innate inflammatory response, as they can directly activate peripheral macrophages and dendritic cells. However, their effect as direct stimulators of B lymphocytes has not been yet fully elucidated. The aim of this study was to examine the molecular mechanisms and cytokine profiles generated following β-glucan stimulation of B lymphocytes, compared with the well-established TLR-9 agonist CpG oligodeoxynucleotide (CpG), and study the participation of β-glucan-stimulated B cells in the innate immune response. In this article, we demonstrate that β-glucan-activated B lymphocytes upregulate proinflammatory cytokines (TNF-α, IL-6, and IL-8). Of interest, β-glucan, unlike CpG, had no effect on B lymphocyte proliferation or IgM production. When compared with CpG (TLR9 agonist), β-glucan-activated cells secreted significantly higher levels of IL-8. Furthermore, IL-8 secretion was partially mediated by Dectin-1 and required SYK, MAPKs, and the transcription factors NF-κB and AP-1. Moreover, we observed that conditioned media from β-glucan-stimulated B lymphocytes elicited neutrophil chemotaxis. These studies suggest that β-glucan-activated B lymphocytes have an important and novel role in fungal innate immune responses.
Copyright © 2015 by The American Association of Immunologists, Inc.
Figures


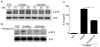
References
-
- Gerondakis S, Grumont RJ, Banerjee A. Regulating B-cell activation and survival in response to TLR signals. Immunol Cell Biol. 2007;85:471–475. - PubMed
-
- Fillatreau S. Cytokine-producing B cells as regulators of pathogenic and protective immune responses. Ann Rheum Dis. 2013;72:ii80–84. - PubMed
-
- Yehudai D, Snir A, Peri R, Halasz K, Haj T, Odeh M, Kessel A. B cell-activating factor enhances interleukin-6 and interleukin-10 production by ODN-activated human B cells. Scand J Immunol. 2012;76:371–377. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

