A randomized trial of the efficacy of artesunate and three quinine regimens in the treatment of severe malaria in children at the Ebolowa Regional Hospital, Cameroon
- PMID: 26520401
- PMCID: PMC4628329
- DOI: 10.1186/s12936-015-0948-0
A randomized trial of the efficacy of artesunate and three quinine regimens in the treatment of severe malaria in children at the Ebolowa Regional Hospital, Cameroon
Abstract
Background: Severe malaria is a medical emergency with high mortality in children below 5 years of age especially in sub-Saharan Africa. Recently, quinine has been replaced by artesunate as the first-line drug in the treatment of severe malaria in Cameroon. No local data are yet available on the efficacy of artesunate with respect to the different quinine regimens used in this setting. This study was undertaken at the Ebolowa Regional Hospital (ERH), which is located in a region of perennial transmission of malaria.
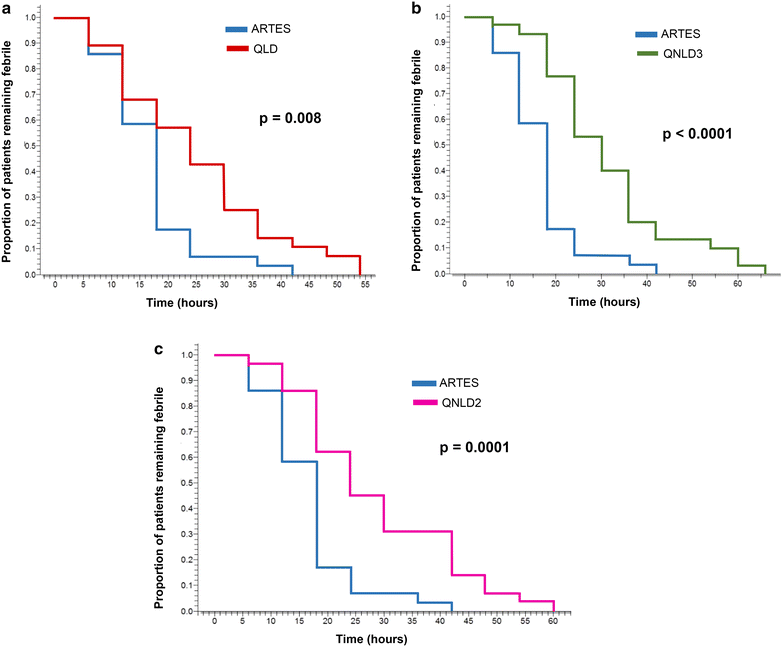
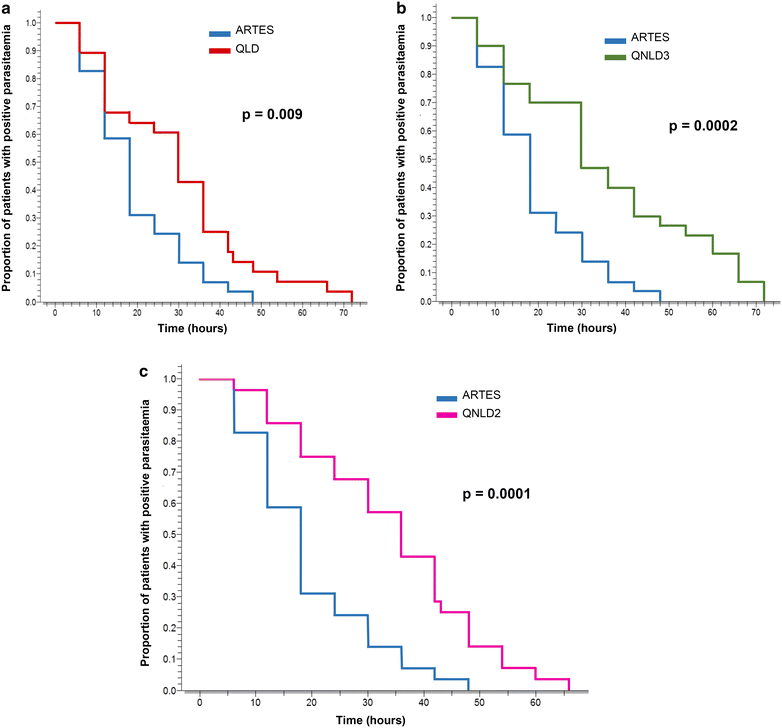
Methods: This was a randomized, open-label trial in children aged 3 months to 15 years, admitted in the hospital with severe malaria due to Plasmodium falciparum confirmed on microscopy after informed parental consent. Patients were randomized into four groups. Group 1 (ARTES) received parenteral artesunate at 2.4 mg/kg at H0, H12, H24 and then once daily; Group 2 (QLD) received a loading dose of quinine base at 16.6 mg/kg followed 8 hours later by an eight-hourly maintenance dose of 8.3 mg/kg quinine base; Group 3 (QNLD3) received 8.3 mg/kg quinine base every 8 hours; and, Group 4 (QNLD2) received 12.5 mg/kg quinine base every 12 h. All patients invariably received a minimum of 24 h parenteral treatment, then, oral drugs were prescribed. The endpoints were fever clearance time, time to sit unsupported, time to eat, parasite clearance time, and parasitaemia reduction rate at H24. Survival analysis was used to compare the outcomes.
Results: One-hundred and sixteen patients completed the study: 29 in ARTES arm, 28 in QLD arm, 30 in QNLD3 arm, and 29 in QNLD2 arm. There was no major differences in baseline characteristics in the treatment groups. On analysis of endpoints, fever clearance time and parasite clearance time were significantly shorter for artesunate-treated patients than for quinine-treated patients. Parasitaemia reduction rate at H24 was also significantly higher for artesunate. Time to sit unsupported and time to eat were shorter with artesunate, but the difference was not statistically significant.
Conclusion: Artesunate is more effective than quinine in the treatment of severe malaria in Cameroonian children.
Figures

References
-
- United Nations. Millennium development goals: Report 2010. New York: United Nations; 2010. http://www.un.org/millenniumgoals/pdf/MDG%20Report%202010%20En%20r15%20-...
-
- Malaria report 2013. ISBN 978 92 4 156469 4 (NLM classification: WC 765).
-
- WHO. Guidelines for the treatment of malaria. Geneva: World Health Organization; 2010. http://www.who.int/malaria/publications/atoz/9789241547925/en/. - PubMed
-
- Institut de la Statistique (INS) Enquête Démographique et de Santé du Cameroun. Calverton: INS et ORC Macro; 2011.
-
- Ministry of Public Health. Guidelines for the management of malaria in Cameroon. Yaounde; 2013.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

