The effects of a 2-h trial of high-flow oxygen by nasal cannula versus Venturi mask in immunocompromised patients with hypoxemic acute respiratory failure: a multicenter randomized trial
- PMID: 26521922
- PMCID: PMC4629403
- DOI: 10.1186/s13054-015-1097-0
The effects of a 2-h trial of high-flow oxygen by nasal cannula versus Venturi mask in immunocompromised patients with hypoxemic acute respiratory failure: a multicenter randomized trial
Abstract
Introduction: In immunocompromised patients, acute respiratory failure (ARF) is associated with high mortality, particularly when invasive mechanical ventilation (IMV) is required. In patients with severe hypoxemia, high-flow nasal oxygen (HFNO) therapy has been used as an alternative to delivery of oxygen via a Venturi mask. Our objective in the present study was to compare HFNO and Venturi mask oxygen in immunocompromised patients with ARF.
Methods: We conducted a multicenter, parallel-group randomized controlled trial in four intensive care units. Inclusion criteria were hypoxemic ARF and immunosuppression, defined as at least one of the following: solid or hematological malignancy, steroid or other immunosuppressant drug therapy, and HIV infection. Exclusion criteria were hypercapnia, previous IMV, and immediate need for IMV or noninvasive ventilation (NIV). Patients were randomized to 2 h of HFNO or Venturi mask oxygen.
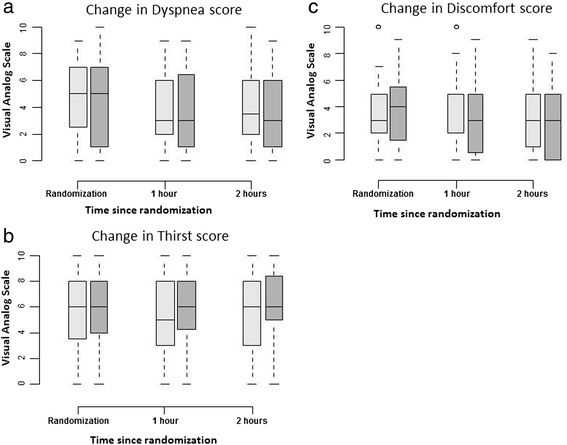
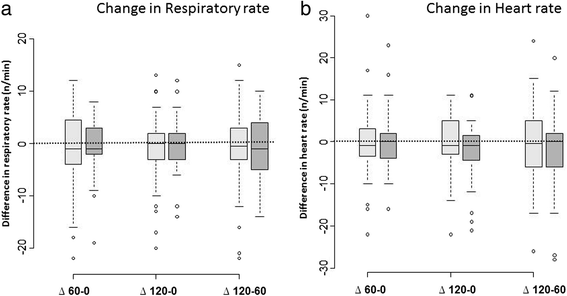
Results: The primary endpoint was a need for IMV or NIV during the 2-h oxygen therapy period. Secondary endpoints were comfort, dyspnea, and thirst, as assessed hourly using a 0-10 visual analogue scale. We randomized 100 consecutive patients, including 84 with malignancies, to HFNO (n = 52) or Venturi mask oxygen (n = 48). During the 2-h study treatment period, 12 patients required IMV or NIV, and we found no significant difference between the two groups (15 % with HFNO and 8 % with the Venturi mask, P = 0.36). None of the secondary endpoints differed significantly between the two groups.
Conclusions: In immunocompromised patients with hypoxemic ARF, a 2-h trial with HFNO improved neither mechanical ventilatory assistance nor patient comfort compared with oxygen delivered via a Venturi mask. However, the study was underpowered because of the low event rate and the one-sided hypothesis.
Trial registration: ClinicalTrials.gov identifier: NCT02424773 . Registered 20 April 2015.
Figures

References
-
- Azoulay E, Mokart D, Pène F, Lambert J, Kouatchet A, Mayaux J, et al. Outcomes of critically ill patients with hematologic malignancies: prospective multicenter data from France and Belgium—a Groupe de Recherche Respiratoire en Reanimation Onco-Hématologique study. J Clin Oncol. 2013;31:2810–8. doi: 10.1200/JCO.2012.47.2365. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

