Long Non Coding RNA MALAT1 Promotes Tumor Growth and Metastasis by inducing Epithelial-Mesenchymal Transition in Oral Squamous Cell Carcinoma
- PMID: 26522444
- PMCID: PMC4629155
- DOI: 10.1038/srep15972
Long Non Coding RNA MALAT1 Promotes Tumor Growth and Metastasis by inducing Epithelial-Mesenchymal Transition in Oral Squamous Cell Carcinoma
Abstract
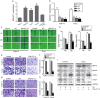
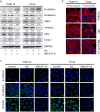
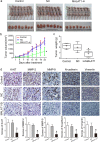
The prognosis of advanced oral squamous cell carcinoma (OSCC) patients remains dismal, and a better understanding of the underlying mechanisms is critical for identifying effective targets with therapeutic potential to improve the survival of patients with OSCC. This study aims to clarify the clinical and biological significance of metastasis-associated long non-coding RNA, metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) in OSCC. We found that MALAT1 is overexpressed in OSCC tissues compared to normal oral mucosa by real-time PCR. MALAT1 served as a new prognostic factor in OSCC patients. When knockdown by small interfering RNA (siRNA) in OSCC cell lines TSCCA and Tca8113, MALAT1 was shown to be required for maintaining epithelial-mesenchymal transition (EMT) mediated cell migration and invasion. Western blot and immunofluorescence staining showed that MALAT1 knockdown significantly suppressed N-cadherin and Vimentin expression but induced E-cadherin expression in vitro. Meanwhile, both nucleus and cytoplasm levels of β-catenin and NF-κB were attenuated, while elevated MALAT1 level triggered the expression of β-catenin and NF-κB. More importantly, targeting MALAT1 inhibited TSCCA cell-induced xenograft tumor growth in vivo. Therefore, these findings provide mechanistic insight into the role of MALAT1 in regulating OSCC metastasis, suggesting that MALAT1 is an important prognostic factor and therapeutic target for OSCC.
Figures


References
-
- Warnakulasuriya S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol 45, 309–316 (2009). - PubMed
-
- Leemans C. R., Braakhuis B. J. & Brakenhoff R. H. The molecular biology of head and neck cancer. Nat Rev Cancer 11, 9–22 (2011). - PubMed
-
- Polyak K. & Weinberg R. A. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer 9, 265–273 (2009). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

