mTORC1 directly phosphorylates and activates ERα upon estrogen stimulation
- PMID: 26522726
- PMCID: PMC4853282
- DOI: 10.1038/onc.2015.414
mTORC1 directly phosphorylates and activates ERα upon estrogen stimulation
Abstract
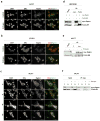
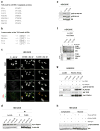
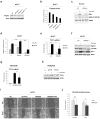
Breast cancer is the leading cause of cancer-related deaths among women. Approximately 75% of breast cancers are estrogen receptor-α (ERα) positive, underscoring the dependence of cancer cells on estrogen for growth and survival. Patients treated with endocrine therapy often develop resistance, either de novo or acquired, which in some cases is caused by aberrations within the growth factor signaling pathways. The mechanistic target of rapamycin complex 1 (mTORC1) has emerged as a critical node in estrogenic signaling. We have previously shown that mTORC1 can phosphorylate and activate ERα on S167 via its effector-the 40S ribosomal S6 kinase 1 (S6K1). Presently, we have uncovered a direct link between mTORC1 and ERα. We found that ERα binds to regulatory-associated protein of mTOR (Raptor) and causes it to translocate to the nucleus upon estrogen stimulation. In addition, we identified mTOR as the kinase that phosphorylates ERα on S104/106 and activates transcription of ER target genes. Our findings show a direct link between mTORC1 and ERα, which further implicates mTORC1 signaling in the pathogenesis of ER-positive breast cancer and provides rationale for FDA-approved use of mTORC1 inhibitors in combination with endocrine agents for treatment of this disease.
Conflict of interest statement
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

