Mechanistic Studies Lead to Dramatically Improved Reaction Conditions for the Cu-Catalyzed Asymmetric Hydroamination of Olefins
- PMID: 26522837
- PMCID: PMC4768687
- DOI: 10.1021/jacs.5b10219
Mechanistic Studies Lead to Dramatically Improved Reaction Conditions for the Cu-Catalyzed Asymmetric Hydroamination of Olefins
Abstract
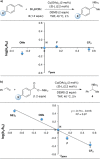
Enantioselective copper(I) hydride (CuH)-catalyzed hydroamination has undergone significant development over the past several years. To gain a general understanding of the factors governing these reactions, kinetic and spectroscopic studies were performed on the CuH-catalyzed hydroamination of styrene. Reaction profile analysis, rate order assessment, and Hammett studies indicate that the turnover-limiting step is regeneration of the CuH catalyst by reaction with a silane, with a phosphine-ligated copper(I) benzoate as the catalyst resting state. Spectroscopic, electrospray ionization mass spectrometry, and nonlinear effect studies are consistent with a monomeric active catalyst. With this insight, targeted reagent optimization led to the development of an optimized protocol with an operationally simple setup (ligated copper(II) precatalyst, open to air) and short reaction times (<30 min). This improved protocol is amenable to a diverse range of alkene and alkyne substrate classes.
Conflict of interest statement
The authors declare no competing financial interest.
Figures








References
-
-
For general references on the construction of amines, see:
- Lawrence S. A.Amines; Cambridge University Press: Cambridge, 2006.
- Ricci A.Modern Amination Methods; Wiley: Weinheim, 2008.
- Nugent T. C.Chiral Amine Synthesis; Wiley: Weinheim, 2010.
- Li W.; Zhang X.. Stereoselective Formation of Amines; Springer: Berlin, 2014.
-
-
-
For general reviews, see:
- Coman S. M.; Parvulescu V. I. Org. Process Res. Dev. 2015, 19, 1327.10.1021/acs.oprd.5b00010. - DOI
- Lee A. V.; Schafer L. L. Eur. J. Inorg. Chem. 2007, 2007, 2245.10.1002/ejic.200700036. - DOI
- Hultzsch K. C. Adv. Synth. Catal. 2005, 347, 367.10.1002/adsc.200404261. - DOI
- Hong S.; Marks T. J. Acc. Chem. Res. 2004, 37, 673.10.1021/ar040051r. - DOI - PubMed
-
-
-
For representative examples of other methods of hydroamination (i.e., base-catalyzed, Brønsted acid-catalyzed, radical-based, and pericyclic reaction-based), see:
- Seayad J.; Tillack A.; Hartung C. G.; Beller M. Adv. Synth. Catal. 2002, 344, 795.10.1002/1615-4169(200209)344:8<795::AID-ADSC795>3.0.CO;2-Q. - DOI
- Schlummer B.; Hartwig J. F. Org. Lett. 2002, 4, 1471.10.1021/ol025659j. - DOI - PubMed
- Anderson L. L.; Arnold J.; Bergman R. G. J. Am. Chem. Soc. 2005, 127, 14542.10.1021/ja053700i. - DOI - PMC - PubMed
- Shapiro N. D.; Rauniyar V.; Hamilton G. L.; Wu J.; Toste F. D. Nature 2011, 470, 245.10.1038/nature09723. - DOI - PMC - PubMed
- Lin J.-S.; Yu P.; Huang L.; Zhang P.; Tan B.; Liu X.-Y. Angew. Chem., Int. Ed. 2015, 54, 7847.10.1002/anie.201501762. - DOI - PubMed
- Guin J.; Mück-Lichtenfield C.; Grimme S.; Studer A. J. Am. Chem. Soc. 2007, 129, 4498.10.1021/ja0692581. - DOI - PubMed
- Nguyen T. M.; Nicewicz D. A. J. Am. Chem. Soc. 2013, 135, 9588.10.1021/ja4031616. - DOI - PMC - PubMed
- Musacchio A. J.; Nguyen L. Q.; Beard G. H.; Knowles R. R. J. Am. Chem. Soc. 2014, 136, 12217.10.1021/ja5056774. - DOI - PubMed
- Beauchemin A. M.; Moran J.; Lebrun M.-E.; Séguin C.; Dimitrijevic E.; Zhang L.; Gorelsky S. I. Angew. Chem., Int. Ed. 2008, 47, 1410.10.1002/anie.200703495. - DOI - PubMed
- Brown A. R.; Uyeda C.; Brotherton C. A.; Jacobsen E. A. J. Am. Chem. Soc. 2013, 135, 6747.10.1021/ja402893z. - DOI - PMC - PubMed
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

