Group 2 innate lymphoid cells license dendritic cells to potentiate memory TH2 cell responses
- PMID: 26523868
- PMCID: PMC4685755
- DOI: 10.1038/ni.3294
Group 2 innate lymphoid cells license dendritic cells to potentiate memory TH2 cell responses
Abstract
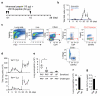
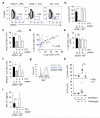
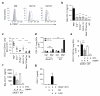
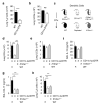
Rapid activation of memory CD4(+) T helper 2 (TH2) cells during allergic inflammation requires their recruitment into the affected tissue. Here we demonstrate that group 2 innate lymphoid (ILC2) cells have a crucial role in memory TH2 cell responses, with targeted depletion of ILC2 cells profoundly impairing TH2 cell localization to the lungs and skin of sensitized mice after allergen re-challenge. ILC2-derived interleukin 13 (IL-13) is critical for eliciting production of the TH2 cell-attracting chemokine CCL17 by IRF4(+)CD11b(+)CD103(-) dendritic cells (DCs). Consequently, the sentinel function of DCs is contingent on ILC2 cells for the generation of an efficient memory TH2 cell response. These results elucidate a key innate mechanism in the regulation of the immune memory response to allergens.
Figures



Comment in
-
Immune memory: ILC2s drive allergen recall.Nat Rev Immunol. 2016 Feb;16(2):72-3. doi: 10.1038/nri.2016.10. Epub 2016 Jan 25. Nat Rev Immunol. 2016. PMID: 26806480 No abstract available.
References
-
- Lambrecht BN, Hammad H. The immunology of asthma. Nat Immunol. 2014;16(1):45–56. - PubMed
-
- McKenzie AN, Spits H, Eberl G. Innate lymphoid cells in inflammation and immunity. Immunity. 2014;41(3):366–374. - PubMed
-
- Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H, et al. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature. 2010;463(7280):540–544. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

