Conformational control of DNA target cleavage by CRISPR-Cas9
- PMID: 26524520
- PMCID: PMC4859810
- DOI: 10.1038/nature15544
Conformational control of DNA target cleavage by CRISPR-Cas9
Abstract
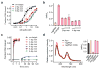
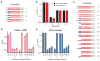
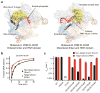
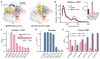
Cas9 is an RNA-guided DNA endonuclease that targets foreign DNA for destruction as part of a bacterial adaptive immune system mediated by clustered regularly interspaced short palindromic repeats (CRISPR). Together with single-guide RNAs, Cas9 also functions as a powerful genome engineering tool in plants and animals, and efforts are underway to increase the efficiency and specificity of DNA targeting for potential therapeutic applications. Studies of off-target effects have shown that DNA binding is far more promiscuous than DNA cleavage, yet the molecular cues that govern strand scission have not been elucidated. Here we show that the conformational state of the HNH nuclease domain directly controls DNA cleavage activity. Using intramolecular Förster resonance energy transfer experiments to detect relative orientations of the Cas9 catalytic domains when associated with on- and off-target DNA, we find that DNA cleavage efficiencies scale with the extent to which the HNH domain samples an activated conformation. We furthermore uncover a surprising mode of allosteric communication that ensures concerted firing of both Cas9 nuclease domains. Our results highlight a proofreading mechanism beyond initial protospacer adjacent motif (PAM) recognition and RNA-DNA base-pairing that serves as a final specificity checkpoint before DNA double-strand break formation.
Figures






References
-
- Doudna JA, Charpentier E. Genome editing. The new frontier of genome engineering with CRISPR-Cas9. Science. 2014;346:1258096. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

