Differential responses to lithium in hyperexcitable neurons from patients with bipolar disorder
- PMID: 26524527
- PMCID: PMC4742055
- DOI: 10.1038/nature15526
Differential responses to lithium in hyperexcitable neurons from patients with bipolar disorder
Erratum in
-
Erratum: Differential responses to lithium in hyperexcitable neurons from patients with bipolar disorder.Nature. 2016 Feb 11;530(7589):242. doi: 10.1038/nature16182. Epub 2015 Nov 25. Nature. 2016. PMID: 26605530 No abstract available.
Abstract
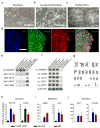
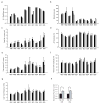
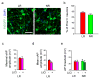
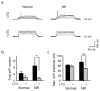
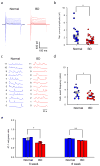
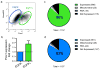
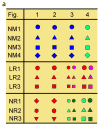
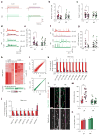
Bipolar disorder is a complex neuropsychiatric disorder that is characterized by intermittent episodes of mania and depression; without treatment, 15% of patients commit suicide. Hence, it has been ranked by the World Health Organization as a top disorder of morbidity and lost productivity. Previous neuropathological studies have revealed a series of alterations in the brains of patients with bipolar disorder or animal models, such as reduced glial cell number in the prefrontal cortex of patients, upregulated activities of the protein kinase A and C pathways and changes in neurotransmission. However, the roles and causation of these changes in bipolar disorder have been too complex to exactly determine the pathology of the disease. Furthermore, although some patients show remarkable improvement with lithium treatment for yet unknown reasons, others are refractory to lithium treatment. Therefore, developing an accurate and powerful biological model for bipolar disorder has been a challenge. The introduction of induced pluripotent stem-cell (iPSC) technology has provided a new approach. Here we have developed an iPSC model for human bipolar disorder and investigated the cellular phenotypes of hippocampal dentate gyrus-like neurons derived from iPSCs of patients with bipolar disorder. Guided by RNA sequencing expression profiling, we have detected mitochondrial abnormalities in young neurons from patients with bipolar disorder by using mitochondrial assays; in addition, using both patch-clamp recording and somatic Ca(2+) imaging, we have observed hyperactive action-potential firing. This hyperexcitability phenotype of young neurons in bipolar disorder was selectively reversed by lithium treatment only in neurons derived from patients who also responded to lithium treatment. Therefore, hyperexcitability is one early endophenotype of bipolar disorder, and our model of iPSCs in this disease might be useful in developing new therapies and drugs aimed at its clinical treatment.
Conflict of interest statement
The authors declare no competing financial interests.
Figures






Comment in
- Neuroscientist. 2016 Apr;22(2):106
-
Psychiatric disorders: Modelling lithium responsiveness in a dish.Nat Rev Neurosci. 2015 Dec;16(12):702. doi: 10.1038/nrn4062. Nat Rev Neurosci. 2015. PMID: 26585797 No abstract available.
-
Reprogramming psychiatry: stem cells and bipolar disorder.Lancet. 2016 Feb 27;387(10021):823-5. doi: 10.1016/S0140-6736(16)00235-X. Epub 2016 Feb 26. Lancet. 2016. PMID: 26972063 No abstract available.
References
-
- Sharma R, Markar HR. Mortality in affective disorder. J Affect Disord. 1994;31:91–96. - PubMed
-
- Andreazza AC, Young LT. The neurobiology of bipolar disorder: identifying targets for specific agents and synergies for combination treatment. Int J Neuropsychopharmacol. 2014;17:1039–1052. - PubMed
-
- Chang A, Li PP, Warsh JJ. cAMP-Dependent protein kinase (PKA) subunit mRNA levels in postmortem brain from patients with bipolar affective disorder (BD) Brain Res Mol Brain Res. 2003;116:27–37. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

