Cost-effectiveness of new antiviral regimens for treatment-naïve U.S. veterans with hepatitis C
- PMID: 26524695
- PMCID: PMC4718749
- DOI: 10.1002/hep.28327
Cost-effectiveness of new antiviral regimens for treatment-naïve U.S. veterans with hepatitis C
Abstract
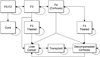
Recently approved, interferon-free medication regimens for treating hepatitis C are highly effective, but extremely costly. We aimed to identify cost-effective strategies for managing treatment-naïve U.S. veterans with new hepatitis C medication regimens. We developed a Markov model with 1-year cycle length for a cohort of 60-year-old veterans with untreated genotype 1 hepatitis C seeking treatment in a typical year. We compared using sofosbuvir/ledipasvir or ombitasvir/ritonavir/paritaprevir/dasabuvir to treat: (1) any patient seeking treatment; (2) only patients with advanced fibrosis or cirrhosis; or (3) patients with advanced disease first and healthier patients 1 year later. The previous standard of care, sofosbuvir/simeprevir or sofosbuvir/pegylated interferon/ribavirin, was included for comparison. Patients could develop progressive fibrosis, cirrhosis, or hepatocellular carcinoma, undergo transplantation, or die. Complications were less likely after sustained virological response. We calculated the incremental cost per quality-adjusted life year (QALY) and varied model inputs in one-way and probabilistic sensitivity analyses. We used the Veterans Health Administration perspective with a lifetime time horizon and 3% annual discounting. Treating any patient with ombitasvir-based therapy was the preferred strategy ($35,560; 14.0 QALYs). All other strategies were dominated (greater costs/QALY gained than more effective strategies). Varying treatment efficacy, price, and/or duration changed the preferred strategy. In probabilistic sensitivity analysis, treating any patient with ombitasvir-based therapy was cost-effective in 70% of iterations at a $50,000/QALY threshold and 65% of iterations at a $100,000/QALY threshold.
Conclusion: Managing any treatment-naïve genotype 1 hepatitis C patient with ombitasvir-based therapy is the most economically efficient strategy, although price and efficacy can impact cost-effectiveness. It is economically unfavorable to restrict treatment to patients with advanced disease or use a staged treatment strategy. (Hepatology 2016;63:428-436).
© 2015 by the American Association for the Study of Liver Diseases. This article has been contributed to by U.S. Government employees and their work is in the public domain in the USA.
Conflict of interest statement
Figures

References
-
- Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology. 2013;57:1333–1342. - PubMed
-
- Chak E, Talal AH, Sherman KE, Schiff ER, Saab S. Hepatitis C virus infection in USA: an estimate of true prevalence. Liver Int. 2011;31:1090–1101. - PubMed
-
- Wong RJ, Aguilar M, Cheung R, Perumpail RB, Harrison SA, Younossi ZM, Ahmed A. Nonalcoholic Steatohepatitis is the Second Leading Etiology of Liver Disease Among Adults Awaiting Liver Transplantation in the U.S. Gastroenterology. 2014 - PubMed
-
- Davis GL, Albright JE, Cook SF, Rosenberg DM. Projecting future complications of chronic hepatitis C in the United States. Liver Transpl. 2003;9:331–338. - PubMed
-
- Butt AA, Wang X, Moore CG. Effect of hepatitis C virus and its treatment on survival. Hepatology. 2009;50:387–392. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

