Germline and Pluripotent Stem Cells
- PMID: 26525151
- PMCID: PMC4632666
- DOI: 10.1101/cshperspect.a019422
Germline and Pluripotent Stem Cells
Abstract
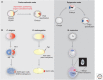
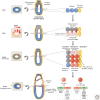
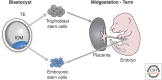
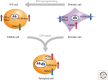
Epigenetic mechanisms play an essential role in the germline and imprinting cycle. Germ cells show extensive epigenetic programming in preparation for the generation of the totipotent state, which in turn leads to the establishment of pluripotent cells in blastocysts. The latter are the cells from which pluripotent embryonic stem cells are derived and maintained in culture. Following blastocyst implantation, postimplantation epiblast cells develop, which give rise to all somatic cells as well as primordial germ cells, the precursors of sperm and eggs. Pluripotent stem cells in culture can be induced to undergo differentiation into somatic cells and germ cells in culture. Understanding the natural cycles of epigenetic reprogramming that occur in the germline will allow the generation of better and more versatile stem cells for both therapeutic and research purposes.
Copyright © 2015 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures




References
-
- Allis CD, Jenuwein T, Reinberg D. 2014. Overview and concepts. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a018739. - DOI
-
- Ancelin K, Lange UC, Hajkova P, Schneider R, Bannister AJ, Kouzarides T, Surani MA. 2006. Blimp1 associates with Prmt5 and directs histone arginine methylation in mouse germ cells. Nat Cell Biol 8: 623–630. - PubMed
-
- Arney KL, Bao S, Bannister AJ, Kouzarides T, Surani MA. 2002. Histone methylation defines epigenetic asymmetry in the mouse zygote. Int J Dev Biol 46: 317–320. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
