Evaluating the QUIT-PRIMO clinical practice ePortal to increase smoker engagement with online cessation interventions: a national hybrid type 2 implementation study
- PMID: 26525410
- PMCID: PMC4630887
- DOI: 10.1186/s13012-015-0336-8
Evaluating the QUIT-PRIMO clinical practice ePortal to increase smoker engagement with online cessation interventions: a national hybrid type 2 implementation study
Abstract
Background: Effective web-assisted tobacco interventions (WATIs) have been underutilized by smokers; moreover, despite practice guideline recommendations, clinical teams do not routinely refer smokers to WATIs. Our goal was to test a clinical practice innovation, an ePortal designed to change practice and patient behavior. Our hypotheses were that the integrated system would result in increased smoker referrals, with an automated follow-up system resulting in more smoker registrations and finally augmentations of the WATI would result in more smokers quitting at 6 months.
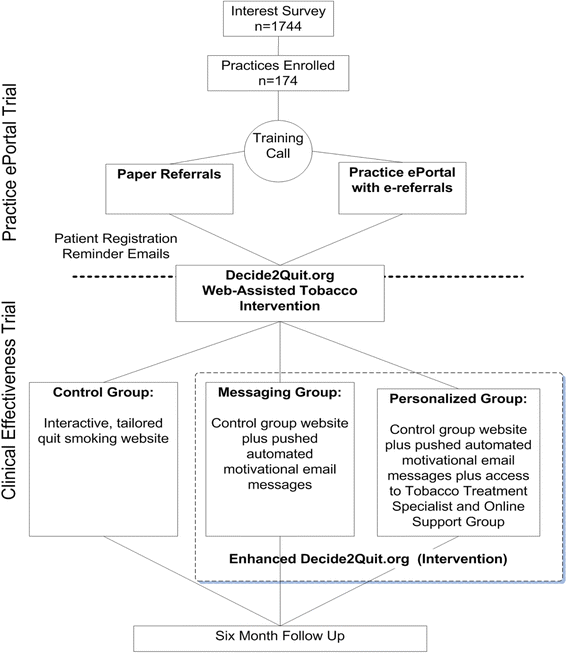
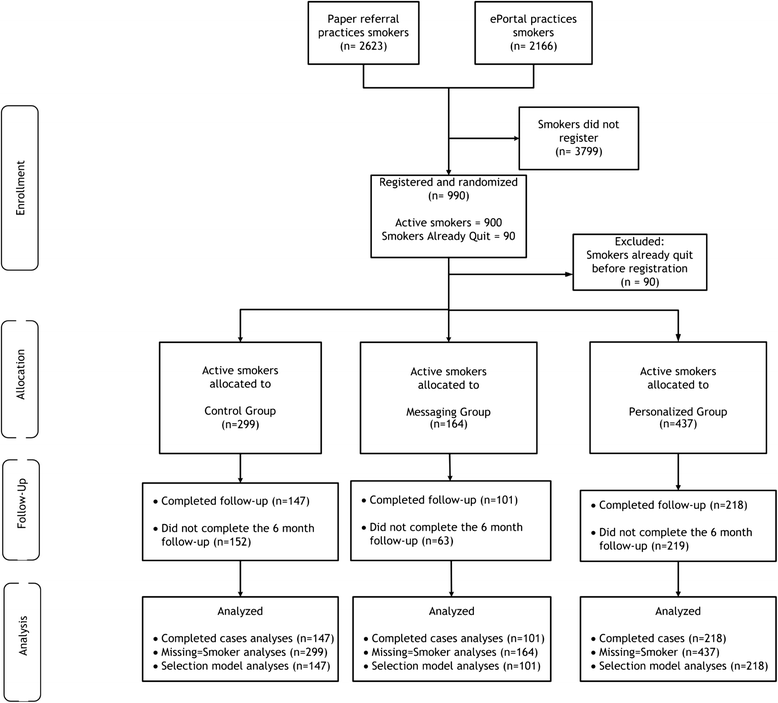
Methods: Practice ePortal Implementation Trial: Practices (n = 174) were randomized to an online practice ePortal with an "e-referral tool" to the WATI (e-referred smokers received automated email reminders from the practice) and with practice feedback reports with patient tracking and practice-to-patient secure messaging versus comparison (a paper "referral prescription"). Implementation success was measured by the number of smokers referred and smokers registering. Clinical Effectiveness Trial: To estimate the effectiveness of the WATI components on 6-month smoking cessation, registered smokers were randomized into three groups: a state-of-the-art tailored WATI control [control], the WATI enhanced with proactive, pushed tailored email motivational messaging (messaging), and the WATI with messaging further enhanced with personal secure messaging with a tobacco treatment specialist and an online support group (personalized).
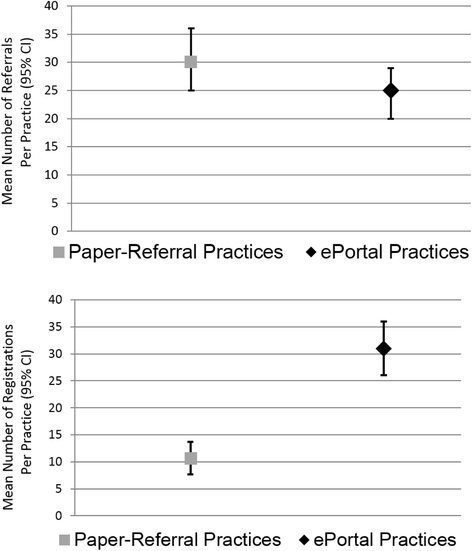
Results: Practice ePortal Trial results: A total of 4789 smokers were referred. The mean smokers referred per practice was not statistically different by group (ePortal 24.89 (SD 22.29) versus comparison 30.15 (SD 25.45), p = 0.15). The e-referral portal implementation program resulted in nearly triple the rate of smoker registration (31 % of all smokers referred registered online) versus comparison (11 %, p < 0.001). Clinical Effectiveness Trial results: Active smokers randomized to the personalized group had a 6-month cessation rate of 25.2 %, compared with the messaging group (26.7 %) and the control (17 %). Next, when using an inverse probability weighted selection model to account for attrition, those randomized to the two groups that received motivational messaging (messaging or personalized) were more likely to quit than those in the control (p = 0.04).
Conclusions: Among all smokers referred, the e-referral resulted in nearly threefold greater registrants (31 %) than paper (11 %). The practice ePortal smokers received multiple reminders (increasing registration opportunities), and the practices could track patient progress. The result was more smokers registering and, thus, more cessation opportunities. Combining the proactive referral and the WATI resulted in higher rates of smoking cessation.
Trial registration: Web-delivered Provider Intervention for Tobacco Control (QUIT-PRIMO) - a randomized controlled trial: NCT00797628 .
Figures

References
-
- Stead LF, Lancaster T. Combined pharmacotherapy and behavioural interventions for smoking cessation. Cochrane Database Syst Rev. 2012;10:CD008286. - PubMed
-
- Stead LF, Bergson G, Lancaster T. Physician advice for smoking cessation. Cochrane Database Syst Rev. 2008(2):CD000165. doi:10.1002/14651858.CD000165.pub3. - PubMed
-
- The Clinical Practice Guideline Treating Tobacco Use and Dependence 2008 Update Panel L, and Staff. A clinical practice guideline for treating tobacco use and dependence: 2008 update: A U.S. Public Health Service Report. Am J Prev Med. 2008; 35(2):158–176. http://dx.doi.org/10.1016/j.amepre.2008.04.009. - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

