Bath additives for the treatment of childhood eczema (BATHE): protocol for multicentre parallel group randomised trial
- PMID: 26525422
- PMCID: PMC4636671
- DOI: 10.1136/bmjopen-2015-009575
Bath additives for the treatment of childhood eczema (BATHE): protocol for multicentre parallel group randomised trial
Abstract
Introduction: Bath emollients are widely prescribed for childhood eczema, yet evidence of their benefits over direct application of emollients is lacking. Objectives To determine the clinical and cost-effectiveness of adding bath emollient to the standard management of eczema in children
Design: Pragmatic open 2-armed parallel group randomised controlled trial.
Setting: General practitioner (GP) practices in England and Wales.
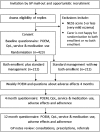
Participants: Children aged over 12 months and less than 12 years with eczema, excluding inactive or very mild eczema (5 or less on Nottingham Eczema Severity Scale).
Interventions: Children will be randomised to either bath emollients plus standard eczema care or standard eczema care only.
Outcome measures: Primary outcome is long-term eczema severity, measured by the Patient-Oriented Eczema Measure (POEM) repeated weekly for 16 weeks. Secondary outcomes include: number of eczema exacerbations resulting in healthcare consultations over 1 year; eczema severity over 1 year; disease-specific and generic quality of life; medication use and healthcare resource use; cost-effectiveness. Aiming to detect a mean difference between groups of 2.0 (SD 7.0) in weekly POEM scores over 16 weeks (significance 0.05, power 0.9), allowing for 20% loss to follow-up, gives a total sample size of 423 children. We will use repeated measures analysis of covariance, or a mixed model, to analyse weekly POEM scores. We will control for possible confounders, including baseline eczema severity and child's age. Cost-effectiveness analysis will be carried out from a National Health Service (NHS) perspective.
Ethics and dissemination: This protocol was approved by Newcastle and North Tyneside 1 NRES committee 14/NE/0098. Follow-up will be completed in 2017. Findings will be disseminated to participants and carers, the public, dermatology and primary care journals, guideline developers and decision-makers.
Trial registration number: ISRCTN84102309.
Keywords: DERMATOLOGY; PRIMARY CARE.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/
Figures
References
-
- Chamlin SL, Frieden IJ, Williams ML et al. . Effects of atopic dermatitis on young American children and their families. Pediatrics 2004;114:607–11. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical