Repeated dose (28-day) administration of silver nanoparticles of varied size and coating does not significantly alter the indigenous murine gut microbiome
- PMID: 26525505
- PMCID: PMC5023147
- DOI: 10.3109/17435390.2015.1078854
Repeated dose (28-day) administration of silver nanoparticles of varied size and coating does not significantly alter the indigenous murine gut microbiome
Abstract
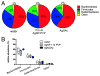
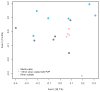
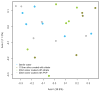
Silver nanoparticles (AgNPs) have been used as antimicrobials in a number of applications, including topical wound dressings and coatings for consumer products and biomedical devices. Ingestion is a relevant route of exposure for AgNPs, whether occurring unintentionally via Ag dissolution from consumer products, or intentionally from dietary supplements. AgNP have also been proposed as substitutes for antibiotics in animal feeds. While oral antibiotics are known to have significant effects on gut bacteria, the antimicrobial effects of ingested AgNPs on the indigenous microbiome or on gut pathogens are unknown. In addition, AgNP size and coating have been postulated as significantly influential towards their biochemical properties and the influence of these properties on antimicrobial efficacy is unknown. We evaluated murine gut microbial communities using culture-independent sequencing of 16S rRNA gene fragments following 28 days of repeated oral dosing of well-characterized AgNPs of two different sizes (20 and 110 nm) and coatings (PVP and Citrate). Irrespective of size or coating, oral administration of AgNPs at 10 mg/kg body weight/day did not alter the membership, structure or diversity of the murine gut microbiome. Thus, in contrast to effects of broad-spectrum antibiotics, repeat dosing of AgNP, at doses equivalent to 2000 times the oral reference dose and 100-400 times the effective in vitro anti-microbial concentration, does not affect the indigenous murine gut microbiome.
Keywords: Antibiotics; in vivo; metastats; microbiome; mothur; mouse; nanomaterials; pyrosequencing; toxicology.
Conflict of interest statement
Declaration of Interests The authors have no conflicts of interest to declare. This study was supported by the National Institute of Environmental Health Sciences of the National Institutes of Health under award number 1U01ES020128-01 as part of the NIEHS Centers for Nanotechnology Health Implications Research Consortium (NCNHIR). The manuscript was reviewed by the NCNHIR consortium prior to submission, however the content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the NCNHIR.
Figures

References
-
- AHMADI F, RAHIMI F. The effect of different levels of nano silver and retention of silver in edible tissues of broilers. World Applied Sciences Journal. 2011;12:01–04.
-
- AHMADI J. Application of different levels of silver nanoparticles in food on the performance and some blood parameters of broiler chickens. World Applied Sciences Journal. 2009;7:24–27.
-
- ANDERSON MJ. A new method for non-parametric multivariate analysis of variance. Austral Ecology. 2001;26:32–46.
-
- ATARASHI K, TANOUE T, HONDA K. Induction of lamina propria Th17 cells by intestinal commensal bacteria. Vaccine. 2010;28:8036–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
