Microbiota-Dependent Hepatic Lipogenesis Mediated by Stearoyl CoA Desaturase 1 (SCD1) Promotes Metabolic Syndrome in TLR5-Deficient Mice
- PMID: 26525535
- PMCID: PMC4670569
- DOI: 10.1016/j.cmet.2015.09.028
Microbiota-Dependent Hepatic Lipogenesis Mediated by Stearoyl CoA Desaturase 1 (SCD1) Promotes Metabolic Syndrome in TLR5-Deficient Mice
Abstract
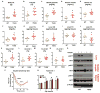
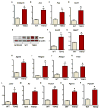
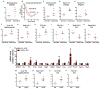
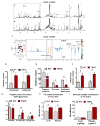
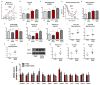
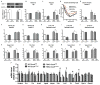
The gut microbiota plays a key role in host metabolism. Toll-like receptor 5 (TLR5), a flagellin receptor, is required for gut microbiota homeostasis. Accordingly, TLR5-deficient (T5KO) mice are prone to develop microbiota-dependent metabolic syndrome. Here we observed that T5KO mice display elevated neutral lipids with a compositional increase of oleate [C18:1 (n9)] relative to wild-type littermates. Increased oleate contribution to hepatic lipids and liver SCD1 expression were both microbiota dependent. Analysis of short-chain fatty acids (SCFAs) and (13)C-acetate label incorporation revealed elevated SCFA in ceca and hepatic portal blood and increased liver de novo lipogenesis in T5KO mice. Dietary SCFAs further aggravated metabolic syndrome in T5KO mice. Deletion of hepatic SCD1 not only prevented hepatic neutral lipid oleate enrichment but also ameliorated metabolic syndrome in T5KO mice. Collectively, these results underscore the key role of the gut microbiota-liver axis in the pathogenesis of metabolic diseases.
Keywords: Toll-like receptor 5; gut bacteria; hepatic neutral lipids; low-grade inflammation; metabolic diseases; monounsaturated fatty acids; short-chain fatty acids.
Copyright © 2015 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors have no conflict of interest.
Figures

Comment in
-
SCFAs Take a Toll En Route to Metabolic Syndrome.Cell Metab. 2015 Dec 1;22(6):954-6. doi: 10.1016/j.cmet.2015.11.006. Cell Metab. 2015. PMID: 26636491
References
-
- Alberts AW, Ferguson K, Hennessy S, Vagelos PR. Regulation of lipid synthesis in cultured animal cells. J Biol Chem. 1974;249:5241–5249. - PubMed
-
- Blottiere HM, Buecher B, Galmiche JP, Cherbut C. Molecular analysis of the effect of short-chain fatty acids on intestinal cell proliferation. Proc Nutr Soc. 2003;62:101–106. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK097865/DK/NIDDK NIH HHS/United States
- R01HL020176/HL/NHLBI NIH HHS/United States
- R01 DK061417/DK/NIDDK NIH HHS/United States
- DK061417/DK/NIDDK NIH HHS/United States
- R01 DK097865-01A1/DK/NIDDK NIH HHS/United States
- DK083890/DK/NIDDK NIH HHS/United States
- R01 DK083890/DK/NIDDK NIH HHS/United States
- R01 HL020176/HL/NHLBI NIH HHS/United States
- R03 DK094864/DK/NIDDK NIH HHS/United States
- DK083275/DK/NIDDK NIH HHS/United States
- T32AI07445/AI/NIAID NIH HHS/United States
- DK59637/DK/NIDDK NIH HHS/United States
- R01HL11264/HL/NHLBI NIH HHS/United States
- R01 ES022186/ES/NIEHS NIH HHS/United States
- K01 DK083275/DK/NIDDK NIH HHS/United States
- ES022186/ES/NIEHS NIH HHS/United States
- U24 DK059637/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

