Doublecortin-Like Kinases Promote Neuronal Survival and Induce Growth Cone Reformation via Distinct Mechanisms
- PMID: 26526391
- PMCID: PMC10069300
- DOI: 10.1016/j.neuron.2015.10.005
Doublecortin-Like Kinases Promote Neuronal Survival and Induce Growth Cone Reformation via Distinct Mechanisms
Abstract
After axotomy, neuronal survival and growth cone re-formation are required for axon regeneration. We discovered that doublecortin-like kinases (DCLKs), members of the doublecortin (DCX) family expressed in adult retinal ganglion cells (RGCs), play critical roles in both processes, through distinct mechanisms. Overexpression of DCLK2 accelerated growth cone re-formation in vitro and enhanced the initiation and elongation of axon re-growth after optic nerve injury. These effects depended on both the microtubule (MT)-binding domain and the serine-proline-rich (S/P-rich) region of DCXs in-cis in the same molecules. While the MT-binding domain is known to stabilize MT structures, we show that the S/P-rich region prevents F-actin destabilization in injured axon stumps. Additionally, while DCXs synergize with mTOR to stimulate axon regeneration, alone they can promote neuronal survival possibly by regulating the retrograde propagation of injury signals. Multifunctional DCXs thus represent potential targets for promoting both survival and regeneration of injured neurons.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures
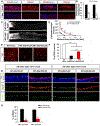
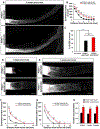


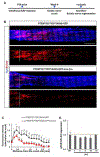

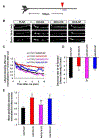

References
-
- Bradke F, Fawcett JW, and Spira ME (2012). Assembly of a new growth cone after axotomy: the precursor to axon regeneration. Nat Rev Neurosci 13, 183–193. - PubMed
-
- Campbell I (2007). Chi-squared and Fisher-Irwin tests of two-by-two tables with small sample recommendations. Stat Med 26, 3661–3675. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

