Normalization of Host Intestinal Mucus Layers Requires Long-Term Microbial Colonization
- PMID: 26526499
- PMCID: PMC4648652
- DOI: 10.1016/j.chom.2015.10.007
Normalization of Host Intestinal Mucus Layers Requires Long-Term Microbial Colonization
Abstract
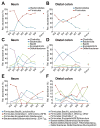
The intestinal mucus layer provides a barrier limiting bacterial contact with the underlying epithelium. Mucus structure is shaped by intestinal location and the microbiota. To understand how commensals modulate gut mucus, we examined mucus properties under germ-free (GF) conditions and during microbial colonization. Although the colon mucus organization of GF mice was similar to that of conventionally raised (Convr) mice, the GF inner mucus layer was penetrable to bacteria-sized beads. During colonization, in which GF mice were gavaged with Convr microbiota, the small intestine mucus required 5 weeks to be normally detached and colonic inner mucus 6 weeks to become impenetrable. The composition of the small intestinal microbiota during colonization was similar to Convr donors until 3 weeks, when Bacteroides increased, Firmicutes decreased, and segmented filamentous bacteria became undetectable. These findings highlight the dynamics of mucus layer development and indicate that studies of mature microbe-mucus interactions should be conducted weeks after colonization.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures
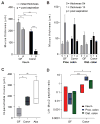
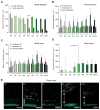
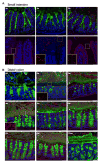
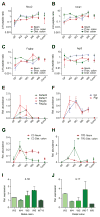
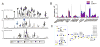
References
-
- Backhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-Bacterial Mutualism in the Human Intestine. Science. 2005;307:1915–1920. - PubMed
-
- Backhed F, Roswall J, Peng Y, Feng Q, Jia H, Kovatcheva-Datchary P, Li Y, Xia Y, Xie H, Zhong H, et al. Dynamics and Stabilization of the Human Gut Microbiome during the First Year of Life. Cell Host Microbe. 2015;17:690–703. - PubMed
-
- Booijink CC, El-Aidy S, Rajilic-Stojanovic M, Heilig HG, Troost FJ, Smidt H, Kleerebezem M, de Vos WM, Zoetendal EG. High temporal and inter-individual variation detected in the human ileal microbiota. Environ Microbiol. 2010;12:3213–3227. - PubMed
-
- Bry L, Falk PG, Midtvedt T, Gordon JI. A model of host-microbial interactions in an open mammalian ecosystem. Science. 1996;273:1380–1383. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

