Game of 'Somes: Protein Destruction for Mycobacterium tuberculosis Pathogenesis
- PMID: 26526503
- PMCID: PMC4698092
- DOI: 10.1016/j.tim.2015.10.001
Game of 'Somes: Protein Destruction for Mycobacterium tuberculosis Pathogenesis
Abstract
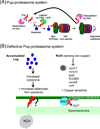
The proteasome system of Mycobacterium tuberculosis is required for causing disease. Proteasomes are multisubunit chambered proteases and, until recently, were only known to participate in adenosine triphosphate (ATP)-dependent proteolysis in bacteria. In this review, we discuss the latest advances in understanding how both ATP-dependent and ATP-independent proteasome-regulated pathways contribute to M. tuberculosis virulence.
Keywords: Mycobacterium tuberculosis; pathogenesis; proteasome; pupylation.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures

References
-
- WHO. World Health Organization; 2014. Tuberculosis fact sheet 2014.
-
- WHO. 2014 Update. World Health Organization; 2014. Multidrug-Resistance Tuberculosis (MDR-TB)
-
- Cambier CJ, Falkow S, Ramakrishnan L. Host evasion and exploitation schemes of Mycobacterium tuberculosis. Cell. 2014;159:1497–1509. - PubMed
-
- Bowman LA, McLean S, Poole RK, Fukuto JM. The diversity of microbial responses to nitric oxide and agents of nitrosative stress close cousins but not identical twins. Adv Microb Physiol. 2011;59:135–219. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

